PTA stands for ‘percutaneous transluminal angioplasty’. PTA balloon catheter has an inflatable balloon at its tip, which is used during a minimally invasive catheterization procedure. The deflated balloon is positioned at the narrowed space and inflated for the short period of time and deflated again to be removed. PTA balloons come in various sizes, lengths, and shapes, depending on the anatomy they are intended to treat. PTA balloon catheters can be used in two ways for the treatment of peripheral vascular lesions. One way is to expand the lumen of an obstructed blood vessel. This method is referred to as the ‘plain-old balloon angioplasty’ (POBA). The second use is for expanding stents for the treatment of vascular blockages.
Global PTA balloon catheter market is estimated to be valued at US$ 1,556.0 million in 2022 and is expected to exhibit a CAGR of 7.1% during the forecast period (2022-2030).
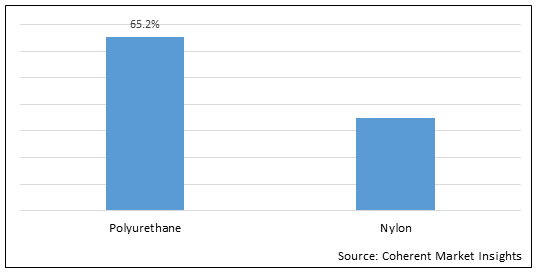
Figure 1. Global PTA Balloon Catheter Market Share (%), by Material Type, 2022
To learn more about this report, Download Free Sample
Increasing preference of polyurethane due to it compatibility is expected to propel growth of the global PTA balloon catheter market over the forecast period.
Polyurethane is considered as the desirable material used for the manufacturing of balloon catheters due to the various advantages provided by it such as
There are several advantages offered by polyurethane balloon such as superior characteristics, including relatively high burst strength and good flexibility, which are expected to drive the segment growth over the forecast period.
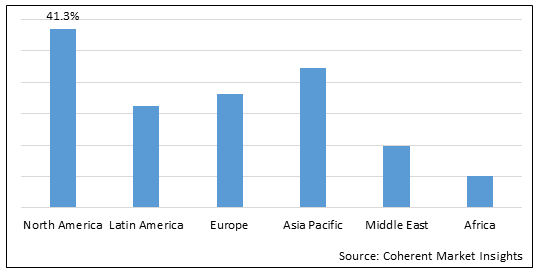
Figure 2. Global PTA Balloon Catheter Market Share (%), by Region, 2022
To learn more about this report, Download Free Sample
Increasing number of inorganic strategies such as product launch and product approval from the U.S. FDA (Food and Drug Administration) by the market players in North America is expected to boost the global PTA balloon catheter market growth over the forecast period.
Increasing number of inorganic strategies such as product launch by the market players in North America is expected to boost the global PTA balloon catheter market growth over the forecast period. For instance, in August 2019, B. Braun Interventional Systems Inc., a medical device company, announced Breakthrough Device Designation for the SeQuent Please ReX drug-coated PTCA (percutaneous transluminal coronary angioplasty) balloon catheter for the treatment of coronary in-stent restenosis (ISR) from the U.S. Food and Drug Administration (FDA).
For instance, on June 2, 2022, Cardio Flow, Inc., a medical device company and developer of minimally invasive peripheral vascular devices to treat peripheral artery disease (PAD), announced that it had received U.S. Food and Drug Administration (FDA) clearance for the company’s FreedomFlow Peripheral Guidewire. The FreedomFlow guidewire is stainless steel core-to-tip design with a fixed distal-spring coil which provide support for diagnostic and therapeutic devices used in treating plaque blockages in arteries both above and below the knee.
PTA Balloon Catheter Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: | US$ 1,556.0 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 7.1% | 2030 Value Projection: | US$ 2,701.0 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Medtronic, Terumo, Cardinal Health, Boston Scientific, AndraTec, Cook Medical, Biotronik, Abbott, Creagh Medical, TriReme Medical, Natec Medical, Surmodics, Inc, B. Braun Melsungen AG, Becton Dickinson and Co, and Acotec Scientific Co Ltd |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global PTA Balloon Catheter Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe and the World Health Organization had declared it a public health emergency on January 30, 2020.
COVID-19 can affect the economy in three main ways: by directly affecting production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries, such as China, India, Saudi Arabia, U.A.E., Egypt, and others, are facing problems with regards to the transportation of drugs from one place to another.
The COVID-19 pandemic and lockdown in various countries across the globe has impacted the financial status of businesses across all sectors. The private healthcare sector is one such sector, which has been majorly impacted by the pandemic. The increase in central-line–associated bloodstream infections and blood culture contamination rates increase is expected to boost the market growth. For instance, in November 2020, National Center for Biotechnology Information published a report which reported that there was a significant increase in central-line–associated bloodstream infections and blood culture contamination rates during the pandemic. Blood culture contamination rates were 19% higher during the COVID-19 period, whereas the CLABSI (central-line–associated bloodstream infections) rate during the pandemic increased by 25% in the U.S.
Thus, impact of the Coronavirus (COVID-19) pandemic had driven growth of the global PTA balloon catheter market during the pandemic.
Global PTA Balloon Catheter Market: Key Developments
The government authorities such as the U.S. Food and Drug Administration, European Commission, and others provide approval for the PTA balloon catheter products in respective regions.
For instance, Surmodics, Inc., a medical device company received the U.S. Food and Drug Administration (FDA) 510(k) and CE Mark clearance for its .014” low-profile percutaneous transluminal angioplasty (PTA) balloon dilation catheter in April 2018.
Furthermore, in September 2021, Medtronic plc. ,a medical device company, announced the CE mark approval and the European launch of the 200mm and 250mm IN.PACT Admiral drug-coated balloons (DCBs). The product is intended to treat long complex femoropopliteal lesions efficiently in patients with peripheral arterial disease.
Global PTA Balloon Catheter Market: Restraint
PTA balloon catheters are regulated by the U.S. Food and Drug Administration in the U.S. The Food and Drug Administration (FDA), the U.S. agency responsible for medical device regulation, issues guidance dedicated to requirements for certain Class II applications. Hence, product recall by U.S. FDA is expected to hamper the market growth.
For instance, in October 2020, the U.S. Food and Administration recalled Class 2 device conquest PTA balloon dilatation catheter offered by Bard Peripheral Vascular Inc., a manufacturer of medical equipments. The product was recalled due to packing issue. The labelling states that the balloon size is 6mm x 40mm, however included a 5mm x 40mm balloon. Dilation catheter was packaged with the wrong size balloon.
In June 2019, Cook Medical’s, a medical device company, product balloon catheter used for percutaneous transluminal angioplasty was recalled by the U.S. FDA. MedWatch, the Food and Drug Administration’s “Safety Information and Adverse Event Reporting Program, reported complaints such as the surgery may be delayed if the balloon burst below the rated burst pressure, requiring another intervention.
Key Players
Major players operating in the global PTA balloon catheter market include Medtronic, Terumo, Cardinal Health, Boston Scientific, AndraTec, Cook Medical, Biotronik, Abbott, Creagh Medical, TriReme Medical, Natec Medical, Surmodics, Inc, B. Braun Melsungen AG, Becton Dickinson and Co, and Acotec Scientific Co Ltd
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients