Pruritus Therapeutics Market Size and Forecast – 2025 – 2032
The Global Pruritus Therapeutics Market size is estimated to be valued at USD 3.6 billion in 2025 and is expected to reach USD 7.8 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 11.4% from 2025 to 2032.
Global Pruritus Therapeutics Market Overview
Pruritus therapeutics focus on the prevention and management of chronic itch, a symptom associated with multiple dermatological, systemic, and neurological conditions. Chronic pruritus significantly affects patient quality of life, leading to sleep disturbances, psychological stress, and impaired daily functioning. The therapeutic landscape includes topical agents such as corticosteroids, antihistamines, and emollients, alongside systemic therapies like immunomodulators, biologics, and neuromodulatory drugs. Recent research emphasizes targeting specific molecular pathways, including interleukin-31 (IL-31) signaling, opioid receptor modulation, and neurokinin-1 receptor inhibition, offering more precise and effective treatment options for patients with refractory pruritus.
The market for pruritus therapeutics is evolving rapidly, driven by increasing prevalence of chronic dermatological disorders and rising awareness of the burden of itch-related conditions. Biologics and novel small molecules are gaining traction due to their targeted mechanisms and improved efficacy over traditional therapies. Moreover, clinical advancements in personalized medicine and patient-centric approaches are expected to expand therapeutic options, improve outcomes, and enhance patient adherence in managing pruritus.
Key Takeaways
In the Therapeutic Class segment, Biologics dominate for strong efficacy in chronic immune-driven pruritus, while small molecule inhibitors grow fastest with convenient oral delivery. Topicals, corticosteroids, and emerging neuromodulators support varied symptom severity and niche treatment needs.
In the indication segment, CKD-aP leads due to high ESRD prevalence, while Atopic Dermatitis grows fastest with rising incidence and new therapies. Psoriasis, cholestatic pruritus, and other immune-linked conditions contribute steady, targeted treatment demand.
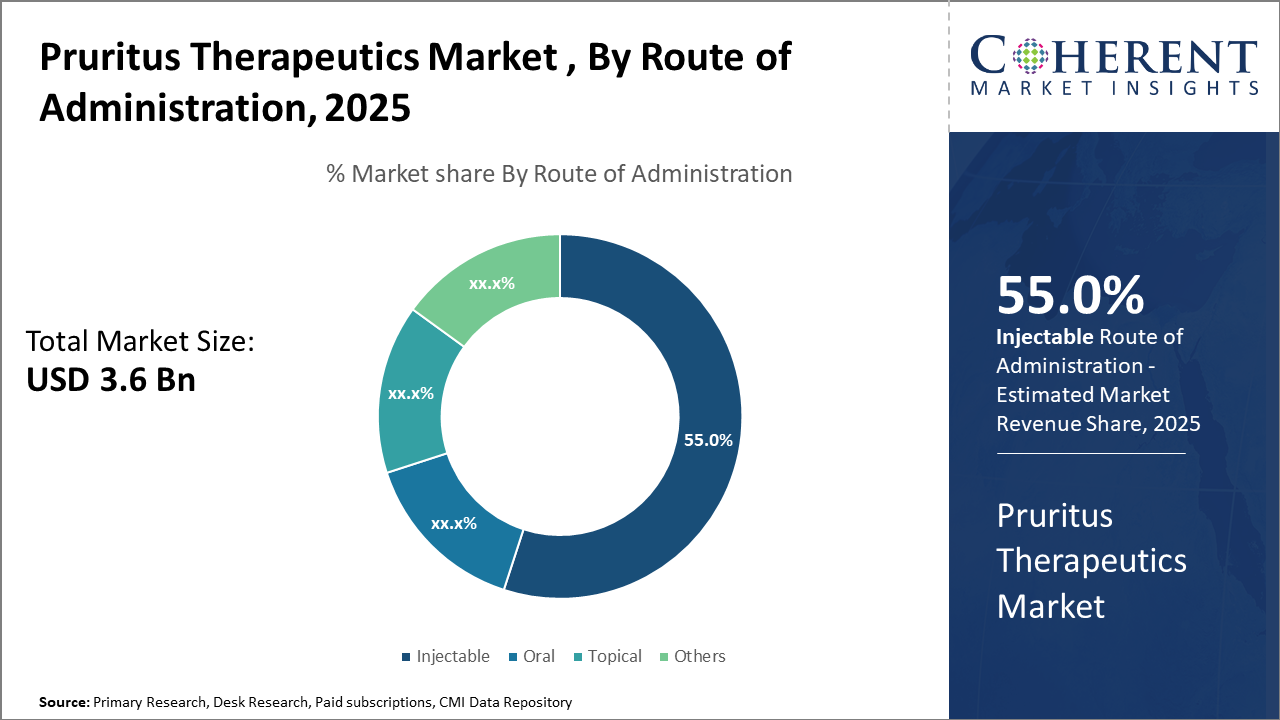
Within Route of Administration segment, Injectables dominate through biologic use requiring parenteral delivery, whereas oral therapies grow fastest with expanding small molecule pipelines. Topicals remain vital for localized relief, and emerging transdermal systems improve patient compliance and access.
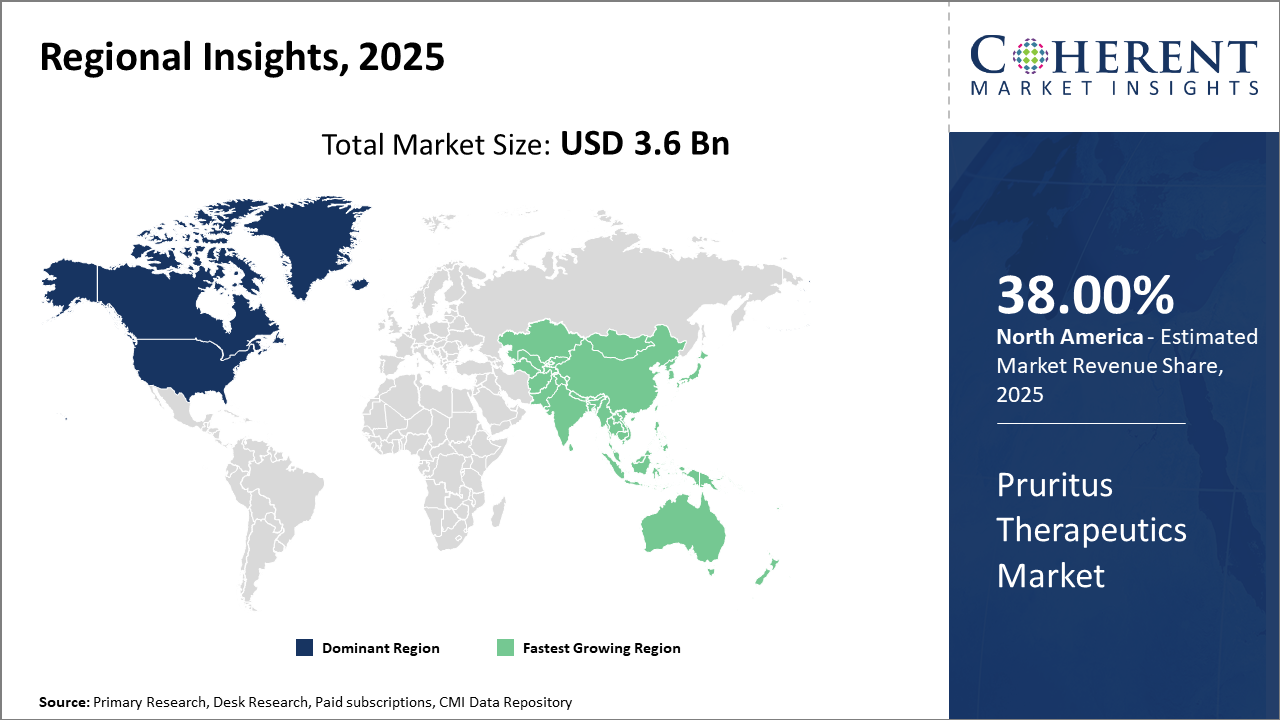
North America dominates pruritus therapeutics with strong healthcare systems and biologic adoption, while Asia Pacific grows fastest driven by rising atopic dermatitis and expanding access. The U.S. leads innovation, and China rapidly advances through investment, partnerships, and increasing disease prevalence.
Pruritus Therapeutics Market Segmentation Analysis
To learn more about this report, Download Free Sample
Pruritus Therapeutics Market Insights, By Route of Administration
Injectables dominate the market due to the extensive use of biologics that require parenteral delivery for optimal bioavailability. Oral therapies are the fastest-growing segment, driven by patient convenience and a strong pipeline of oral small molecule inhibitors. Topical formulations remain crucial for localized itch with minimal systemic effects, while emerging methods such as transdermal patches and microneedles enhance compliance and broaden therapeutic options.
Pruritus Therapeutics Market Insights, By Therapeutic Class
Biologics dominate the pruritus therapeutics market due to their superior efficacy in chronic, immune-mediated conditions, while small molecule inhibitors represent the fastest-growing class, supported by oral convenience and expanding JAK and IL-4/IL-13 inhibitor pipelines. Topical agents remain important for mild to moderate cases, systemic corticosteroids serve short-term needs, and emerging neuromodulators form a niche category.
Pruritus Therapeutics Market Insights, By Indication
CKD-aP dominates due to its high prevalence in ESRD patients requiring specialized therapies, while Atopic Dermatitis is the fastest-growing segment driven by rising global incidence and expanding treatment approvals. Psoriasis and cholestatic pruritus maintain moderate shares with increasing use of targeted agents, and other conditions such as prurigo nodularis and systemic autoimmune disorders contribute to additional niche demand.
Pruritus Therapeutics Market Trends
The pruritus therapeutics market is moving rapidly toward targeted immunotherapies and personalized treatment approaches, driven by advancements in biomarker research and molecular diagnostic assays that enable precise patient profiling.
Precision medicine is gaining momentum, highlighted by the surge in IL-31 and JAK inhibitor approvals in 2024, which reflect growing confidence in mechanism-specific therapies for chronic pruritus.
Digital health adoption is accelerating, with teledermatology platforms improving access to specialist care, particularly for chronic and underserved populations.
Remote monitoring tools and mobile applications are enhancing patient engagement, enabling continuous symptom tracking and more informed clinical decision-making.
Collectively, these trends are reshaping market dynamics by improving treatment accuracy, optimizing care pathways, and elevating long-term patient outcomes.
Pruritus Therapeutics Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Pruritus Therapeutics Market Analysis and Trends
In North America, the dominance in the Pruritus Therapeutics market is driven by robust healthcare expenditures, advanced treatment infrastructure, and early adoption of novel therapies. The U.S., in particular, contributes significantly owing to a high ESRD patient population and favorable reimbursement environments. Horizon Therapeutics and Regeneron have bolstered their presence through well-received biologics, collectively accounting for approximately 38% of global market revenue.
Asia Pacific Pruritus Therapeutics Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth with a CAGR exceeding 14%, driven by increasing prevalence of atopic dermatitis and growing healthcare access in countries like China and India. Government-led initiatives to improve dermatology services and expansion of biopharmaceutical manufacturing have contributed to rapid market development. Companies such as Sun Pharmaceutical Industries and Astellas Pharma are actively expanding their portfolios and market footprint here.
Pruritus Therapeutics Market Outlook for Key Countries
USA Pruritus Therapeutics Market Analysis and Trends
The U.S. market represents the largest share due to the high incidence of chronic kidney disease and atopic dermatitis. Regulatory support for innovative drugs and value-based care models incentivize market players to introduce advanced biologics. Recent approvals of new IL-31 inhibitors have seen adoption rates exceed 20% year-over-year in major medical centers. Leading companies focus on patient-centric clinical trials and expanding specialty clinic networks to solidify market leadership.
China Pruritus Therapeutics Market Analysis and Trends
China’s market is rapidly expanding due to increased healthcare spending and rising awareness of pruritus-associated conditions. The government's emphasis on improving dermatological care coupled with increasing urbanization fuels demand. Local market companies are leveraging partnerships with multinational firms to introduce novel therapies. The market growth is further endorsed by rising incidences of atopic dermatitis and systemic conditions linked to pruritus, accentuated by demographic shifts.
Analyst Opinion
The market is expanding due to rapid growth in targeted therapeutics, particularly biologics with high cytokine specificity. Newly approved IL-31 inhibitors have broadened treatment options and drove a 15% revenue increase from 2023 to 2024. A leading IL-31 receptor antagonist in the U.S. boosted prescription volumes by more than 20% year-over-year, demonstrating strong clinician and patient adoption of advanced therapies.
Patient demand is rising as awareness and diagnosis of pruritus linked to systemic diseases improve. Increasing ESRD prevalence in North America and Europe has accelerated the use of treatments for uremic pruritus, where symptom prevalence exceeds 40%. Consequently, therapies approved for CKD-aP recorded nearly 12% market share growth in 2024.
Pricing strategies are shifting to support premium biologics and traditional treatments. Biologic ASPs rose by 8% in 2024, yet sales volumes increased due to better insurance coverage, improving patient access and sustaining overall market momentum.
Supply-side expansion is visible through increased manufacturing capacity and active clinical pipelines. In 2024, manufacturers grew production throughput by 18%, aligned with rising global demand. More than 25 Phase II/III clinical trials are underway, highlighting continued investment in novel itch pathway therapeutics.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 3.6 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 11.4% | 2032 Value Projection: | USD 7.8 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Horizon Therapeutics plc, Pfizer Inc., Regeneron Pharmaceuticals, Inc., Sanofi S.A., Novartis AG, Astellas Pharma Inc., AbbVie Inc., GlaxoSmithKline plc, UCB S.A., Eli Lilly and Company, Sun Pharmaceutical Industries Ltd., Johnson & Johnson, Amgen Inc., Mundipharma International Limited, F. Hoffmann-La Roche Ltd., Kyowa Kirin Co.Ltd. Bottom of Form | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Pruritus Therapeutics Market Growth Factors
The Pruritus Therapeutics market is primarily driven by the rising prevalence of chronic conditions such as atopic dermatitis and chronic kidney disease, which have amplified the need for advanced treatments with improved safety and efficacy. Increasing awareness of pruritus’ impact on quality of life has further improved diagnosis rates, supporting higher market revenue. Additionally, ongoing investments in biologics research and development of tailored therapies are creating new indications, expanding both the scope and application of pruritus treatments globally.
The adoption of teledermatology and other digital health tools has enhanced patient access, monitoring, and adherence, contributing to market growth. Furthermore, regulatory agencies are increasingly granting fast-track approvals for novel therapies, accelerating product availability. These combined factors are driving strategic expansion and innovation across the pruritus therapeutics landscape.
Pruritus Therapeutics Market Development
In June 2025, Galderma, a leading pure-play dermatology company, announced the launch of two clinical trials evaluating nemolizumab for Systemic Sclerosis (SSc) and Chronic Pruritus of Unknown Origin (CPUO), both conditions with significant unmet medical needs. Nemolizumab is a monoclonal antibody that blocks the IL-31 receptor alpha, preventing IL-31 signaling—a key driver of itch in CPUO and of inflammation and fibrosis in SSc.
Key Players
Leading Companies of the Market
Horizon Therapeutics plc
Pfizer Inc.
Regeneron Pharmaceuticals, Inc.
Sanofi S.A.
Novartis AG
Astellas Pharma Inc.
AbbVie Inc.
GlaxoSmithKline plc
UCB S.A.
Eli Lilly and Company
Sun Pharmaceutical Industries Ltd.
Johnson & Johnson
Amgen Inc.
Mundipharma International Limited
F. Hoffmann-La Roche Ltd.
Kyowa Kirin Co.Ltd.
Several market companies have adopted competitive strategies centered around advancing clinical pipeline robustness and strategic collaborations. For instance, one leading player recently completed a partnership with academic institutions to accelerate IL-31 antagonists' development, leading to expedited regulatory approvals in 2024. Another prominent company leveraged acquisition strategies to augment their biologics portfolio, resulting in a 17% increase in market share in North America over the past year.
Pruritus Therapeutics Market Future Outlook
The Pruritus Therapeutics market is poised for robust growth, driven by rising prevalence of chronic skin and systemic conditions, expanding biologics and small molecule pipelines, and increasing adoption of personalized medicine. Advances in targeted therapies, including IL-31 and JAK inhibitors, alongside digital health tools like teledermatology, are enhancing patient access, adherence, and treatment outcomes. Emerging markets in Asia Pacific and Latin America present new growth opportunities due to improving healthcare infrastructure and awareness. Ongoing R&D, supportive regulatory pathways, and innovations in delivery methods, such as transdermal systems, are expected to sustain long-term market expansion and diversify therapeutic options globally.
Pruritus Therapeutics Market Historical Analysis
The Pruritus Therapeutics market has historically experienced steady growth driven by rising awareness of chronic pruritus and its impact on quality of life. Early market expansion was supported by conventional therapies, including antihistamines, corticosteroids, and topical agents, primarily targeting mild to moderate cases. Over time, increasing prevalence of systemic and dermatological conditions, such as CKD-aP and atopic dermatitis, spurred demand for advanced therapies. The introduction of biologics and small molecule inhibitors in the late 2010s marked a shift toward targeted treatment, improving clinical outcomes and setting the foundation for rapid adoption in hospitals, clinics, and specialty care settings.
Sources
Primary Research Interviews:
Dermatologists and Oncologists
Clinical Pharmacologists
Hospital Pharmacy Directors
Health Information Management (HIM) Specialists
Patient Advocacy Groups
Databases:
ClinicalTrials.gov
PubMed / MEDLINE
Global Health Data Exchange (GHDx)
WHO Global Health Observatory
Magazines & Industry Publications:
Healthcare IT News
Health Data Management
Modern Healthcare
Dermatology Times
Journals:
Journal of Dermatological Treatment
Journal of Clinical Medicine
International Journal of Dermatology
Journal of the American Medical Informatics Association (JAMIA)
Associations & Organizations:
American Academy of Dermatology (AAD)
International Society of Dermatology (ISD)
National Kidney Foundation (NKF)
European Academy of Dermatology and Venereology (EADV)
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients