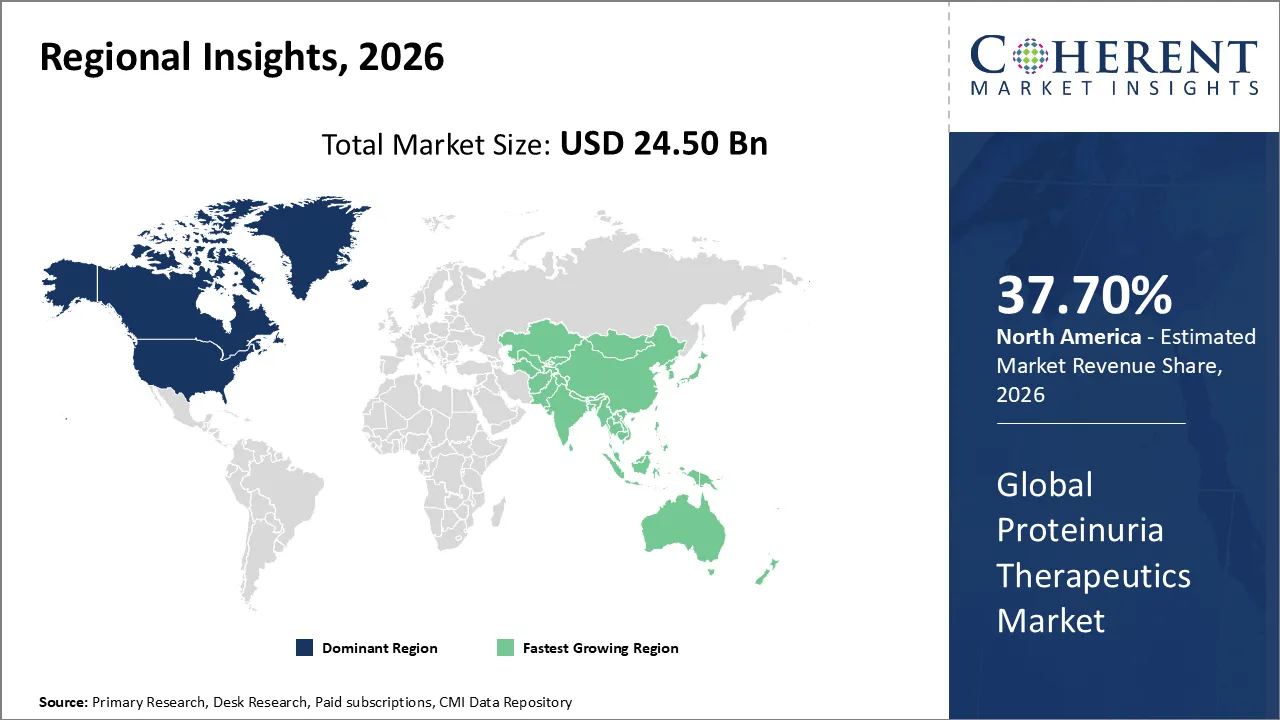
The Proteinuria Therapeutics Market is estimated to be valued at USD 24.50 Bn in 2026 and is expected to reach USD 73.40 Bn by 2033, exhibiting a compound annual growth rate (CAGR) of 17.2% from 2026 to 2033.
The Proteinuria Therapeutics Market develops and delivers treatments for conditions marked by excess protein in the urine, mainly caused by chronic kidney disease, diabetes, and hypertension. Key therapies include ACE inhibitors, ARBs, SGLT2 inhibitors, and newly emerging oral and injectable targeted drugs. The market expands as kidney-related disorders become more prevalent, awareness of early detection grows, drug development advances, and healthcare infrastructure strengthens. Hospitals and outpatient centers drive distribution, guided by clinical protocols and patient demand for effective, long-term care.
|
Current Events |
Description and its impact |
|
Regulatory and Policy Developments |
|
|
Technological and Scientific Innovations |
|
|
Epidemiological and Demographic Trends |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Angiotensin-converting Enzyme (ACE) hold the largest market share of 33.2% in 2026. The Angiotensin-Converting Enzyme (ACE) segment drives the Proteinuria Therapeutics Market by actively slowing kidney damage and reducing proteinuria. Increasing cases of chronic kidney disease, diabetes, and hypertension fuel demand for ACE inhibitors, which control blood pressure while protecting renal function. Physicians follow clinical guidelines and promote early intervention, further boosting their use. Advances in drug formulations and combination therapies improve patient adherence, establishing ACE inhibitors as a central treatment option for the long-term management of proteinuria. For instance, PERINDOPRIL-INDAPAMIDE is prescribed for adults as an initial treatment for mild to moderate essential hypertension. The combination pairs perindopril erbumine, an ACE inhibitor, with indapamide, a chlorosulphamoyl diuretic. Together, these active ingredients work synergistically to effectively lower blood pressure in patients with hypertension.
Oral expected to hold largest market share of 61.3% in 2026. The oral segment actively drives the Proteinuria Therapeutics Market by offering convenient, easy-to-administer, and long-term treatment options. Patients rely on oral medications to manage chronic conditions such as CKD, diabetes, and hypertension, which improves adherence and ensures consistent therapy. The availability of generics and affordable alternatives expands access, while hospitals and outpatient centers efficiently deliver these treatments. Continuous innovations in oral formulations and the launch of targeted agents further boost their use, establishing oral therapies as a major force in proteinuria management. For instance, Travere Therapeutics announced that the U.S. Food and Drug Administration has granted accelerated approval to FILSPARI™ (sparsentan) to reduce proteinuria in adults with primary IgA nephropathy at risk of rapid disease progression.
Hospitals pharmacies acquired the prominent market share of 34.4% in 2026. Hospital pharmacies actively drive the Proteinuria Therapeutics Market by providing patients with chronic kidney disease, diabetes, and hypertension timely access to essential medications. They administer complex therapies, including specialized oral and injectable treatments, while ensuring close monitoring and patient adherence. By integrating pharmacy services with clinical care, hospitals coordinate effective management of proteinuria. Rising patient demand, advanced healthcare infrastructure, and efficient in-house drug distribution further reinforce hospital pharmacies as a central force in delivering successful proteinuria treatments.
To learn more about this report, Download Free Sample
North America dominates the overall market with an estimated share of 37.7% in 2026. The North America Proteinuria Therapeutics Market grows as the rising prevalence of chronic kidney disease, diabetes, and hypertension drives demand for effective proteinuria management. Advanced healthcare infrastructure, widespread early diagnostic practices, and adherence to clinical guidelines support this expansion. Pharmaceutical companies actively develop and introduce innovative oral and injectable therapies, broadening treatment options. Increasing patient awareness, strong insurance coverage, and a focus on integrated care continue to influence market dynamics and reinforce North America’s role as a leading region in proteinuria therapeutics. For instance, Tarpeyo™ (budesonide) is now available to treat adults with primary IgA nephropathy by reducing proteinuria in patients at risk of rapid disease progression.
The Asia Pacific Proteinuria Therapeutics Market expands as the rising incidence of diabetes, hypertension, and chronic kidney disease increases demand for effective treatments. Patients actively seek care due to growing awareness of early diagnosis and improved access to healthcare services. Investments in healthcare infrastructure, the launch of advanced oral and injectable therapies, and engagement by domestic and multinational pharmaceutical companies drive market growth. Governments implement supportive initiatives, hospitals expand networks, and healthcare providers adopt clinical guidelines, collectively shaping the region’s proteinuria therapeutics landscape.
The United States Proteinuria Therapeutics Market grows as the high prevalence of chronic kidney disease, diabetes, and hypertension increases the demand for effective proteinuria management. Patients take advantage of early screening programs and advanced diagnostic tools that allow timely intervention. Pharmaceutical companies actively launch innovative oral and injectable therapies, while hospitals and specialty clinics deliver comprehensive care. Strong insurance coverage, robust healthcare infrastructure, and adherence to clinical guidelines enhance treatment access, establishing the United States as a leading market for proteinuria therapeutics. For instance, Aurinia Pharmaceuticals launched Time Is Nephrons, the first lupus nephritis awareness campaign for healthcare professionals. Lupus nephritis, a serious complication of systemic lupus erythematosus, causes kidney inflammation that leads to proteinuria.
The India Proteinuria Therapeutics Market grows as rising cases of diabetes, hypertension, and chronic kidney disease increase demand for effective treatments. Patients actively seek timely care due to greater awareness of early diagnosis and improved access to healthcare services. Pharmaceutical companies launch innovative oral and injectable therapies, while hospitals and clinics deliver integrated management. Government initiatives, expanding healthcare infrastructure, and the adoption of clinical guidelines further enhance treatment availability, establishing India as a rapidly emerging market in proteinuria therapeutics.
Oral medications are increasingly favored for managing proteinuria due to their convenience, suitability for long-term therapy, and ease of adherence. Patients with chronic conditions like diabetes, hypertension, and CKD prefer oral options over injectables. Pharmaceutical companies are investing in innovative oral formulations, including extended-release and combination therapies, which improve treatment compliance. Hospitals, clinics, and outpatient centers actively distribute these drugs, strengthening the oral segment as a major driver of market growth and patient-centered care.
Hospitals and specialty clinics remain key channels for proteinuria treatment, particularly for severe cases and complex comorbidities. These settings enable integrated care, including diagnostics, therapy administration, and patient monitoring. Hospital pharmacies actively coordinate medication access, ensuring adherence to clinical guidelines. The growing network of hospitals, combined with rising patient demand for specialist care, reinforces these channels’ importance, driving both treatment uptake and awareness of advanced therapies across diverse patient populations in proteinuria management.
The market presents opportunities for pharmaceutical companies to develop advanced oral and injectable treatments that target proteinuria more effectively. Patients increasingly demand therapies with fewer side effects, improved adherence, and long-term kidney protection. Innovation in combination therapies and extended-release formulations can enhance patient compliance. Companies that focus on these novel solutions can capture a growing segment of chronic disease patients, establishing leadership in both established and emerging markets for proteinuria therapeutics.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 24.50 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 17.2% | 2033 Value Projection: | USD 73.40 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
GlaxoSmithKline plc, Chinook Therapeutics Inc., Novartis International AG, AbbVie Inc., Calliditas Therapeutics AB, Travere Therapeutics Inc., Walden Biosciences, Ligand Pharmaceuticals Inc., and Retrophin, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients