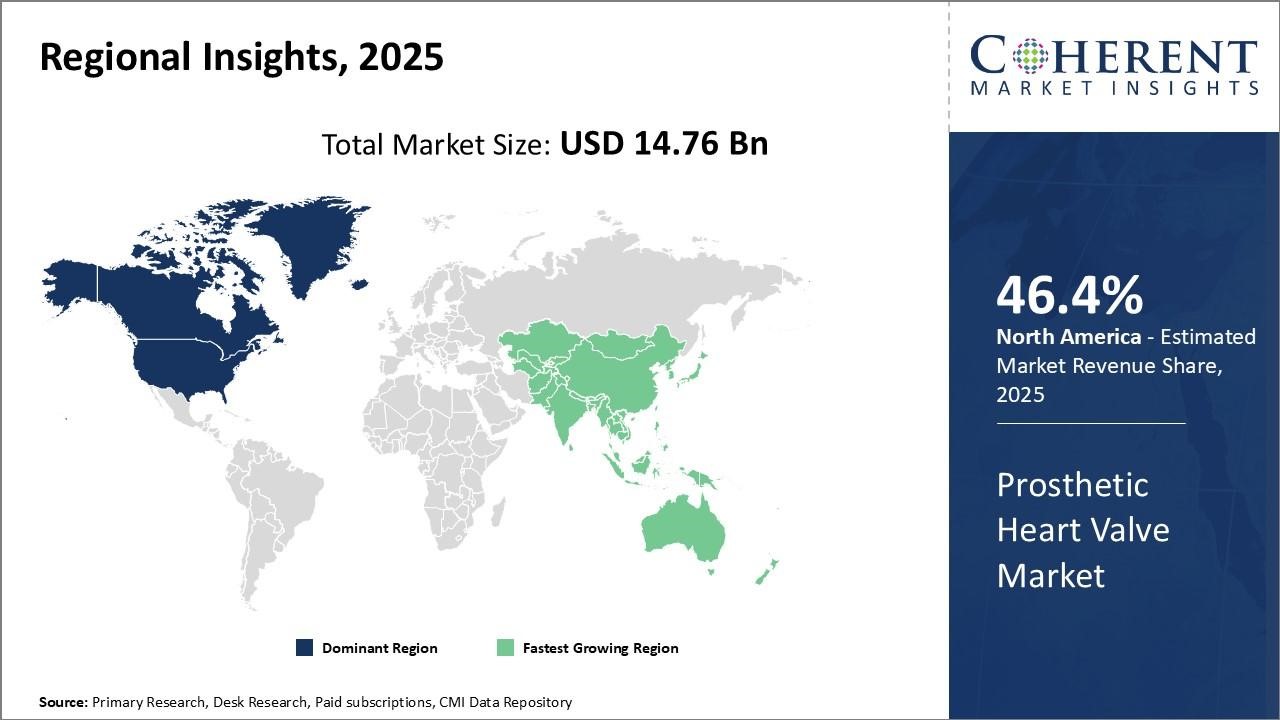
The prosthetic heart valve market is estimated to be valued at USD 14.76 Bn in 2025 and is expected to reach USD 32.04 Bn by 2032, growing at a compound annual growth rate (CAGR) of 11.7% from 2025 to 2032.
To learn more about this report, Download Free Sample
The market is expected to witness positive growth over the forecast period owing to the rising geriatric population worldwide, coupled with the growing prevalence of cardiovascular diseases. Additionally, technological advancements in the field of heart valves, such as the development of transcatheter heart valves and the increasing preference for minimally invasive procedures, are expected to drive the demand for prosthetic heart valves. Overall, increasing investments by governments and players to enhance the healthcare infrastructure will continue aiding the expansion of the prosthetic heart valve market over the coming years.
|
Current Events |
Description and its impact |
|
Aging Demographics and Cardiovascular Disease Burden |
|
|
Regulatory Environment Evolution and Medical Device Oversight |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The position type segment includes the aortic valve, mitral valve, and others. The aortic valve contributes the highest share of the market and is expected to hold 40.2% of the market share in 2024. Aortic stenosis carries grave mortality risks if left untreated, and its prevalence increases sharply with age. Given lifestyle-related risks, rising life expectancies have meant growing incidence rates of severe aortic stenosis worldwide. At the same time, transcatheter aortic valve replacement (TAVR) procedures have revolutionized care for high-risk groups by providing a minimally invasive alternative to conventional surgery. These factors have significantly driven the demand for aortic valve replacements, with TAVR now accounting for the majority of procedures. Additional tailwinds include favorable reimbursement policies supporting TAVR adoption and coordinated care models ensuring timely intervention.
In January 2025, Abbott launched the Navitor Vision in India for treating symptomatic severe aortic stenosis in patients at high or extreme surgical risk. This latest version of the Navitor TAVI/TAVR system features three large Vision markers to enhance visibility and simplify valve deployment, building on the platform’s reputation for stability, performance, and future-ready design.
The valve type segment includes mechanical valves, tissue valves, and transcatheter valves. The mechanical valves segment is anticipated to hold 40.2% of the market share in 2025. Several major players are consistently investing in R&D to develop newer generations of mechanical valves with improved durability and hemodynamic performance. The latest products offer minimized thrombosis risks and employ advanced materials that reduce wear and tear. Additionally, mechanical valves are highly preferred for younger patients since they last decades and eliminate reoperation risks. Recent 3D printing technologies are also enabling customized, patient-specific mechanical heart valves with minimal blood leakage. As newer technological breakthroughs enhance mechanical valves' efficacy and longevity, their adoption rates among cardiovascular specialists remain high for various severities of valvular heart disease.
An article from the National Library of Medicine states that mechanical valves are preferred for certain patients because of their superior long-term durability. Unlike biological valves, which can deteriorate over time, mechanical valves are more likely to last, making them a suitable option for younger patients or those who can safely undergo lifelong anticoagulation therapy, thereby lowering the risk of needing repeat surgeries.
The end user segment includes hospitals, ambulatory surgical centers, catheterization laboratories, and others. Catheterization laboratory contributes the highest share of the prosthetic heart valve market and is projected to hold 39.1% of the market share in 2025. Catheterization Laboratories contributes the highest share of the market, driven by its central role in transcatheter heart procedures. Since their introduction over a decade ago, TAVR and other valve implantation techniques have transformed cardiovascular care.
By avoiding the trauma of open-heart surgery, they provide treatment access for patients previously deemed inoperable. This has led to rapid capacity expansion at catheterization labs nationwide to meet the burgeoning demand. Additionally, labs benefit from strong relationships with cardiologists, cardiac surgeons, and the hospital administration, aiding effective coordination of heart team efforts. As transcatheter options for mitral regurgitation and other applications evolve, catheterization labs will continue promoting early intervention approaches and positively impact valve replacement market growth. For instance, in January 2025, the Department of Cardiology at Christian Medical College (CMC) Vellore unveiled a cutting-edge Cardiac Catheterization Laboratory.
To learn more about this report, Download Free Sample
The North American region has remained the dominant market for prosthetic heart valves globally and is projected to hold 46.4% of the market share in 2025. This can be attributed to cutting-edge research and the strong presence of industry leaders in the region. On the pricing front, North America can command a small premium over other regions due to the brand reputation of valves manufactured by domestic companies. However, with rising healthcare costs, payers are pushing for more affordable options. This has led companies to rationalize portfolios by discontinuing older surgical valve models in favor of their latest transcatheter alternatives. Given the large patient pool in the US, even a small decline in average selling price can have a significant impact on overall revenues for firms.
The Asia Pacific region has emerged as the fastest-growing marketplace for prosthetic heart valves globally. Rapid modernization of healthcare infrastructure and an increase in per capita incomes are propelling growth. Governments in various APAC countries have implemented national health insurance plans to ensure wider access to cardiac treatments. However, with a higher proportion of rheumatic heart disease cases compared to developed markets, the preference remains for cheaper mechanical valves in this region rather than tissue/bioprosthetic alternatives.
The U.S. is the prominent market in North America. The U.S., in particular, houses the headquarters of major players like Edwards Lifesciences, Medtronic, and Abbott that continue to invest heavily in R&D for the development of innovative valve designs. Furthermore, favorable reimbursement policies for transcatheter aortic valve replacement (TAVR) procedures in the country ensure higher adoption rates of newer valve technologies.
Some of the major drivers in the market include the presence of major players, reimbursement policies, and continuous research and innovation. For example, Medtronic has broadened the U.S. launch of its latest self-expanding transcatheter aortic valve replacement (TAVR) device, the Evolut™ FX system. This next-generation valve builds on the existing Evolut platform with new features designed to improve usability and provide more consistent, controlled valve deployment for physicians. This is further adding to the prosthetic heart valve market share.
Canada is the second-largest in the region. The growth is attributed to the publicly funded healthcare system, an increase in the rate of heart diseases, etc. The government in the country also promptly supports innovations in the medical industry. Moreover, collaborations in the industry also lead to the development of cost-effective solutions. For instance, in September 2025, the iValve was rolled out by UBC Okanagan researchers. It is a novel heart valve that combines mechanical durability with tissue-like performance.
China is the largest contributor to the prosthetic heart valve market in the Asia Pacific region. China dominates the market in this region due to the rapidly growing and aging population in the country. This leads to an increase in the prevalence of cardiovascular diseases. Moreover, the manufacturing of prosthetic heart valves within the country has also reduced costs and improved affordability. For instance, in March 2025, a team of surgeons implanted the world’s smallest magnetic artificial heart into a 7-year-old at the Wuhan Union Hospital.
To learn more about this report, Download Free Sample
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 14.76 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 11.7% | 2032 Value Projection: | USD 32.04 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
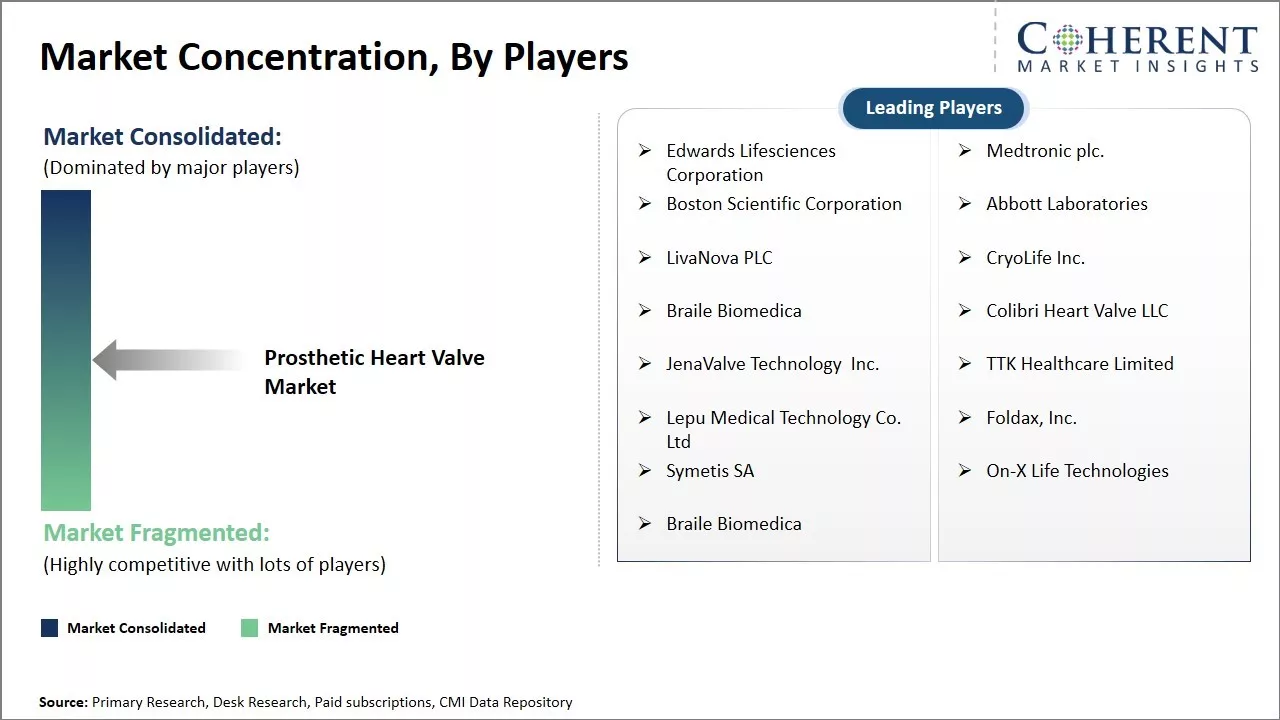
Edwards Lifesciences Corporation, Medtronic plc., Boston Scientific Corporation, Abbott Laboratories, LivaNova PLC, CryoLife Inc., Braile Biomedica, Colibri Heart Valve LLC, JenaValve Technology Inc., TTK Healthcare Limited, Lepu Medical Technology Co. Ltd, Foldax, Inc., Symetis SA, On-X Life Technologies, and Braile Biomedica |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
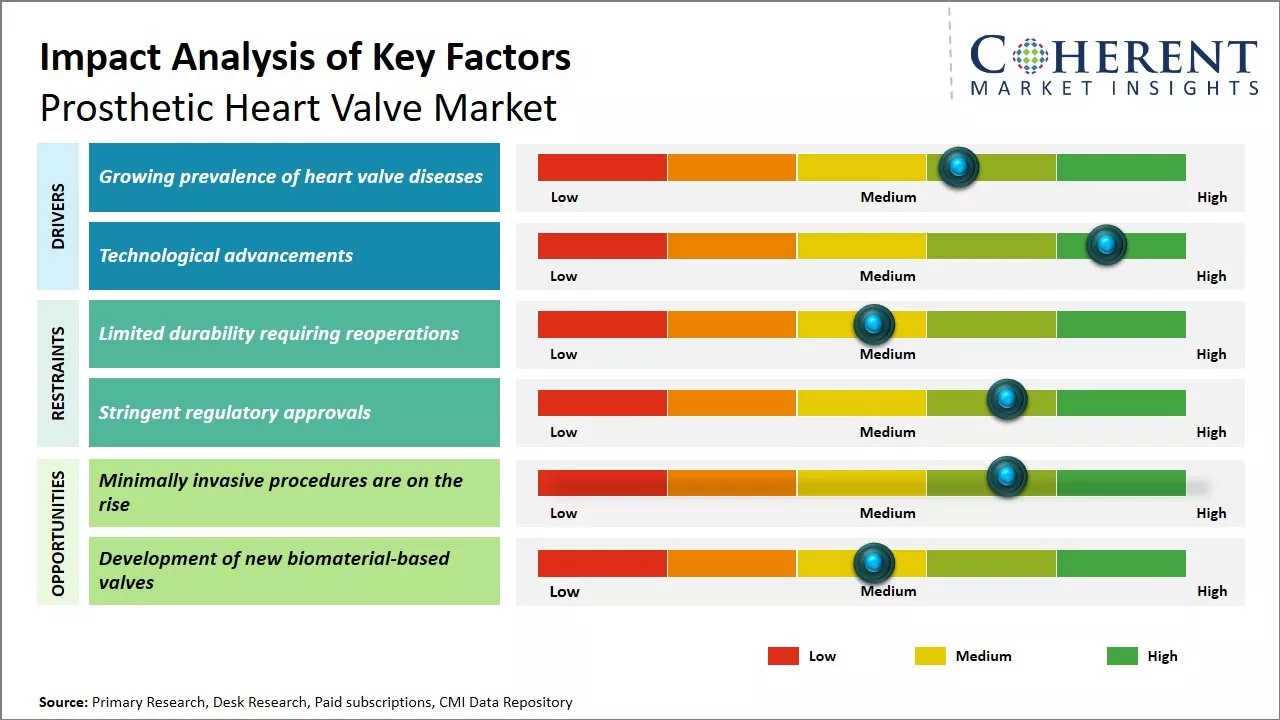
The increasing prevalence of heart valve diseases acts as a key driver for the prosthetic heart valve market. Heart valve diseases traditionally affect elderly populations, with degenerative valve disorders being the most common. However, with changing lifestyles, diseases associated with heart valves are being observed in younger populations as well. Conditions like rheumatic fever, which can damage heart valves, are no longer limited to developing regions.
Another factor contributing to the rising incidence of valve disorders is the growing epidemic of obesity and diabetes across the world. For example, a World Obesity article reports that 1 in 5 adults globally are projected to have obesity by 2025, yet no country is currently on track to meet the established targets for that year. Both these conditions increase the risk of developing heart problems over the long term. As populations in developed economies continue to battle rising obesity, the burden of diabetes and other associated cardiac complications has increased. Early onset of lifestyle diseases means more people entering middle age with damaged heart valves. As populations grow older worldwide, age-related valve defects will affect a greater number of individuals.
Technological advancements represent a key driver propelling the prosthetic heart valve market growth. Continuous R&D efforts are reshaping the valve replacement landscape with new materials, minimally invasive techniques, and transcatheter options. Mechanical heart valves, which were the standard for decades, are now slowly making way for bioprosthetic or tissue valves due to their superior hemodynamic performance and patient acceptance. Engineered tissue and stent construction methods have enhanced the durability of bioprostheses by close to 10-15 years.
Transcatheter aortic valve replacement (TAVR) procedures using catheter-delivered valves have emerged as a less invasive alternative to open-heart surgeries for high-risk patient groups. TAVR systems are innovative wherein that collapsed valve substitutes are delivered to the site via catheters and implanted through small incisions. Their perceived advantages over surgical valves, such as quicker recovery times, are increasing uptake globally. Device manufacturers are striving to develop transcatheter technologies suitable for mitral, tricuspid, and pulmonary valve positions as well. This is further proliferating the prosthetic heart valve market demand.
The growing geriatric population susceptible to valvular heart disease will drive demand. Transcatheter aortic valve replacement minimally invasive procedures are on the rise, allowing the treatment of inoperable patients. Newer bioprosthetic valves could provide durable alternatives to mechanical ones reducing health risks. Developing valves compatible for pediatric patients are expected to create lucrative opportunities for market development over the forecasted period.
*Definition: The prosthetic heart valve market involves the manufacturing and sales of prosthetic heart valves used to replace damaged or diseased valves in patients suffering from valve disorders. These artificial heart valves are surgically implanted to restore the heart's normal pumping function. The key types of prosthetic heart valves available are mechanical heart valves and bioprosthetic or tissue heart valves. The global prosthetic heart valve market has been growing due to the rising geriatric population suffering from valvular heart diseases.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients