Primary Biliary Cirrhosis Drugs Market Size and Forecast – 2025 – 2032
The Global Primary Biliary Cirrhosis Drugs Market size is estimated to be valued at USD 1.35 billion in 2025 and is expected to reach USD 2.12 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 6.3% from 2025 to 2032.
Global Primary Biliary Cirrhosis Drugs Market Overview
PBC drugs include therapeutic formulations aimed at managing chronic autoimmune liver disease by slowing disease progression and reducing bile acid buildup. Key product types include bile acid analogs like ursodeoxycholic acid (UDCA), farnesoid X receptor (FXR) agonists such as obeticholic acid, and emerging treatments like PPAR agonists and immunomodulators. These drugs are available in oral dosage forms and focus on improving liver function and patient quality of life.
Key Takeaways
The ursodeoxycholic acid segment dominates the drug class category with a 53.4% share, buoyed by its well-established efficacy and tolerability profile.
The obeticholic acid segment is rapidly growing, presenting promising opportunities due to clinical advancements.
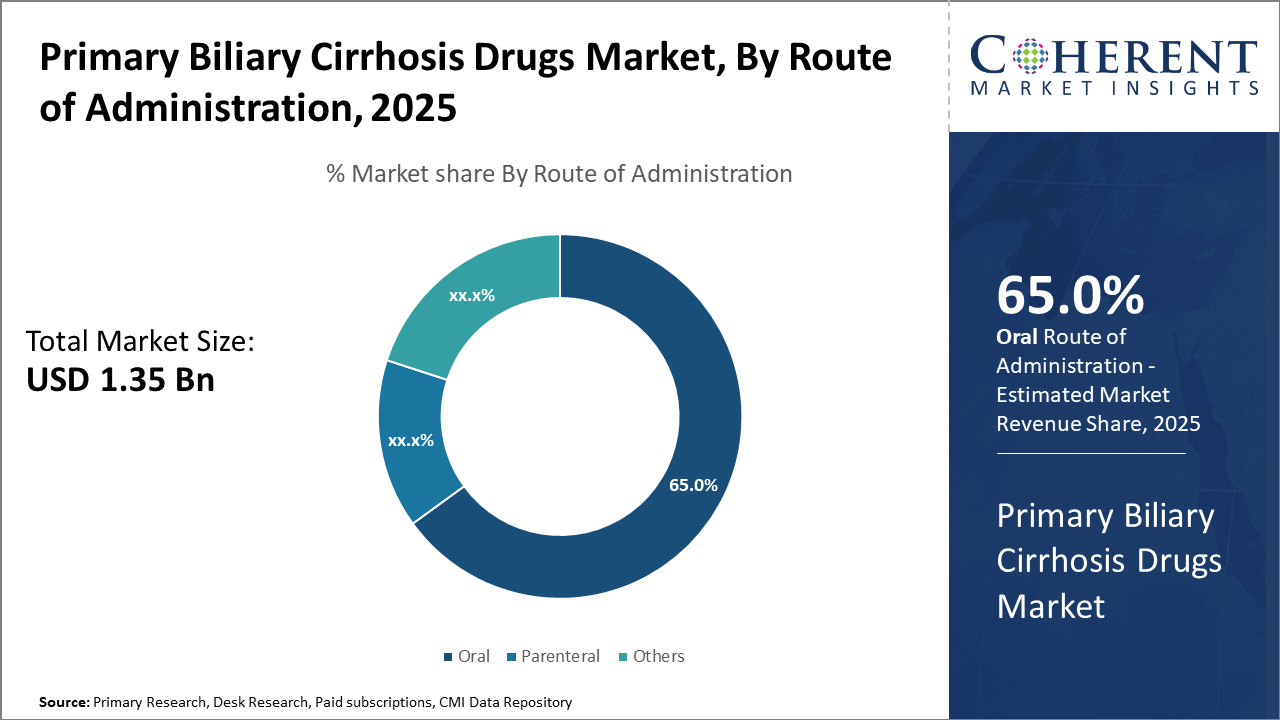
Oral administration leads in the route of delivery at 65%, attributed to patient convenience and higher compliance. Parenteral routes maintain a niche application for specific cases.
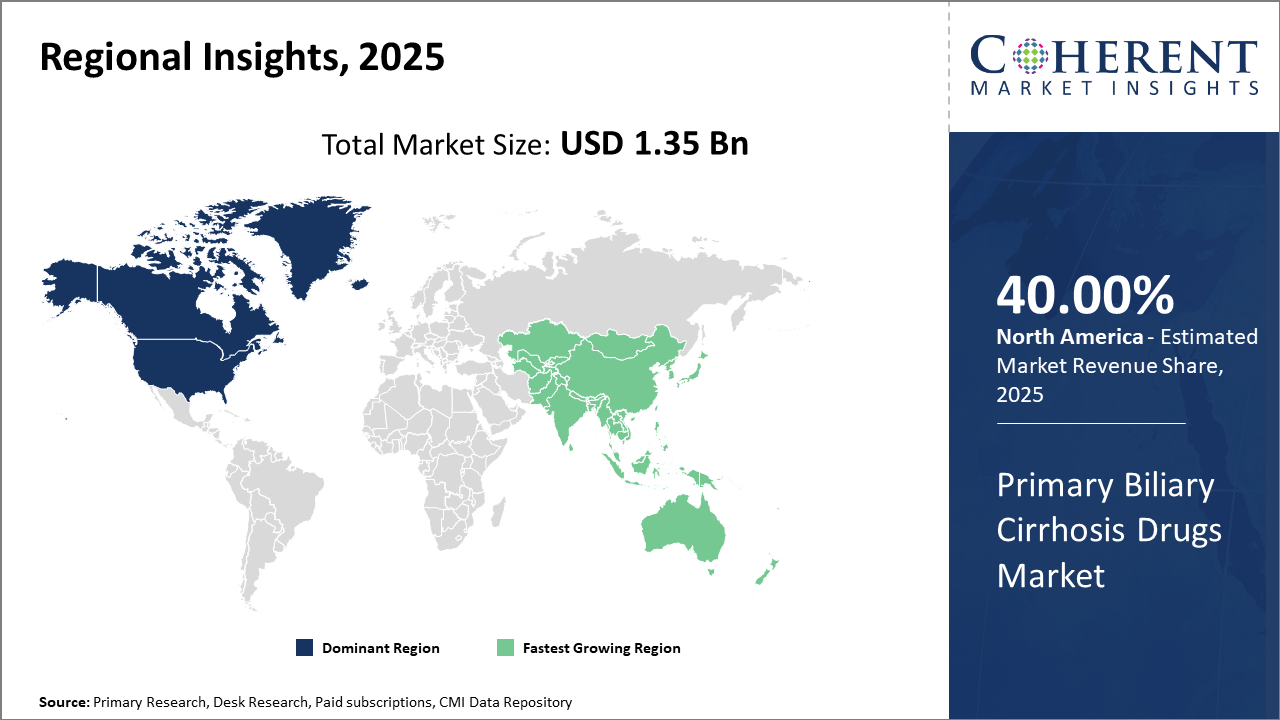
North America continues as the key regional market, accounting for over 40% of market share, driven by advanced healthcare infrastructure and reimbursement frameworks.
Asia Pacific represents the fastest-growing region, exhibiting over 8% CAGR, driven by an expanding patient base and improving healthcare accessibility.
Primary Biliary Cirrhosis Drugs Market Segmentation Analysis
To learn more about this report, Download Free Sample
Primary Biliary Cirrhosis Drugs Market Insights, By Drug Class
Ursodeoxycholic acid dominates the market share, owing to its proven efficacy in delaying disease progression and a favorable tolerability profile, supporting widespread clinical adoption. The obeticholic acid subsegment represents the fastest-growing class, benefiting from regulatory approvals in refractory PBC cases and its improved biochemical response, as evidenced by rising prescription trends in 2024. Fibrates provide adjunct benefits targeting inflammatory pathways but have limited penetration. Immunosuppressants, although less prominent, are gaining traction in combination therapy.
Primary Biliary Cirrhosis Drugs Market Insights, By Route of Administration
Regarding the route of administration, the market encompasses oral, parenteral, and other routes, with oral administration dominating substantially. The convenience, cost-effectiveness, and improved patient adherence associated with oral formulations underscore their market dominance. Parenteral routes remain limited to specialized care settings and for patients with advanced liver disease complications. Emerging formulations aimed at enhancing oral bioavailability and reducing adverse effects will propel growth within this segment.
Primary Biliary Cirrhosis Drugs Market Insights, By Distribution Channel
Hospital pharmacies dominate due to the chronic nature of PBC treatments requiring medical supervision and controlled dispensing. Retail pharmacies cater primarily to maintenance therapy and refill demands but face regulatory and prescription constraints. Online pharmacies are witnessing gradual growth, particularly in regions with developed telemedicine infrastructure, providing improved drug accessibility. Additional channels like specialty pharmacies focus on rare and advanced therapies, augmenting the overall market reach and supporting patient-centric care.
Primary Biliary Cirrhosis Drugs Market Trends
The Primary Biliary Cirrhosis Drugs market is characterized by three significant trends shaping its trajectory.
Firstly, increasing adoption of biomarker-driven therapies enhances treatment personalization, offering superior clinical outcomes and supporting premium pricing strategies.
For instance, studies from 2024 highlight a 12% decrease in disease progression rates when treatment is tailored versus standard protocols.
Secondly, the rising prevalence of autoimmune liver disorders due to lifestyle and environmental factors underscores an uptick in demand for effective PBC therapeutics globally.
Thirdly, digital health technologies facilitate better patient adherence and monitoring, especially in developed markets, improving drug efficacy and patient quality of life.
Primary Biliary Cirrhosis Drugs Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Primary Biliary Cirrhosis Drugs Market Analysis and Trends
In North America, the dominance in the Primary Biliary Cirrhosis Drugs market stems from well-established healthcare infrastructure, robust reimbursement policies, and advanced diagnostic capabilities. The region contributes over 40% to the total industry share. Key players such as AbbVie and Intercept Pharmaceuticals heavily invest in clinical trials and product launch initiatives within the U.S., fortifying market leadership.
Asia Pacific Primary Biliary Cirrhosis Drugs Market Analysis and Trends
The Asia Pacific exhibits the fastest growth in the PBC drugs market, with a CAGR exceeding 8%. Factors such as rising healthcare expenditure, enhanced regulatory frameworks, and growing patient awareness contribute significantly. Emerging economies like China and India are witnessing increased drug imports and local pharmaceutical investments to address unmet clinical needs.
Primary Biliary Cirrhosis Drugs Market Outlook for Key Countries
USA Primary Biliary Cirrhosis Drugs Market Analysis and Trends
The USA's market stands as a critical growth engine for Primary Biliary Cirrhosis Drugs, largely due to early disease detection capabilities and strong payer support. In 2024, prevalence rates increased by 9%, concurrently driving drug sales uplift by over 10%. Companies with strategic patient support programs and clinical innovation, such as Intercept Pharmaceuticals, have reported double-digit revenue growth. Furthermore, FDA approvals of novel immunomodulatory agents signal sustained market dynamism in the country.
Japan Primary Biliary Cirrhosis Drugs Market Analysis and Trends
Japan’s market is bolstered by government-backed healthcare policies addressing autoimmune liver diseases, creating favorable reimbursement conditions for PBC therapies. The country has seen a surge in clinical research investments, with innovative treatments accounting for approximately 30% of total drug revenues by 2024. Leading pharmaceutical companies actively expanding regional portfolios have contributed to robust business growth, capturing an increasing share of the Asia Pacific market.
Analyst Opinion
The demand-side indicators reveal that patient diagnosis rates for primary biliary cirrhosis rose approximately 8.2% annually between 2023 and 2024, leading to a significant increase in treatment initiation and consequently market revenue. For instance, the U.S. saw a 10% rise in new PBC cases reported in 2024 alone, which directly boosted drug adoption rates.
Production capacity for key therapeutic compounds, including ursodeoxycholic acid, expanded by 12% in major pharmaceutical hubs across Europe and North America in 2024, enhancing drug availability and supporting steady market growth.
Pricing trends in the PBC drugs market have exhibited moderate increments, with average treatment cost rises of 4.5% year-over-year between 2023 and 2025, reflecting the introduction of novel, patented drug formulations while maintaining patient affordability.
Import volumes of innovative PBC therapeutics in Asia Pacific surged by 15%, driven by growing healthcare infrastructure and regulatory approvals. This trend illustrates shifting market dynamics where emerging economies increasingly contribute to consumption growth.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 1.35 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.3% | 2032 Value Projection: | USD 2.12 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | AbbVie Inc., Takeda Pharmaceutical Company Ltd., Intercept Pharmaceuticals Inc., Gilead Sciences, Inc., Novartis AG, Pfizer Inc., Sanofi S.A., Merck & Co., Inc., Mitsubishi Tanabe Pharma Corporation, Cipla Ltd., Sun Pharmaceutical Industries Ltd., Dr. Reddy’s Laboratories Ltd. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Primary Biliary Cirrhosis Drugs Market Growth Factors
The rising prevalence of autoimmune liver diseases globally remains the primary growth driver, evidenced by a 7.1% increase in diagnosed PBC cases between 2023 and 2025. Advances in drug formulations, particularly the development of second-generation bile acid analogs, continue to enhance therapeutic efficacy, driving higher adoption rates. Increasing government initiatives offering reimbursement schemes for chronic liver conditions in North America and Europe have significantly diminished patient financial burden, encouraging long-term treatment compliance. Furthermore, expanding clinical trials evaluating combination therapies for PBC are expected to catalyze innovative drug launches, stimulating market growth dynamics by creating diversified treatment landscapes.
Primary Biliary Cirrhosis Drugs Market Development
In August 2024, the U.S. Food and Drug Administration (FDA) approved seladelpar (brand name: Livdelzi) for the treatment of PBC. This approval allows seladelpar to be used in combination with ursodeoxycholic acid (UDCA) for patients who have not responded adequately to UDCA, or as a monotherapy for those unable to tolerate UDCA. Seladelpar has demonstrated efficacy in reducing pruritus and interleukin-31 levels in PBC patients.
On 10 June 2024, Ipsen announced that the U.S. Food and Drug Administration (FDA) has granted accelerated approval for Iqirvo® (elafibranor) 80 mg tablets for the treatment of primary biliary cholangitis (PBC). The therapy is approved for use in combination with ursodeoxycholic acid (UDCA) in adults who show an inadequate response to UDCA, or as monotherapy for patients who cannot tolerate UDCA. Eligible patients in the U.S. may now be prescribed Iqirvo immediately.
Key Players
Leading Companies of the Market
AbbVie Inc.
Takeda Pharmaceutical Company Ltd.
Intercept Pharmaceuticals Inc.
Gilead Sciences, Inc.
Novartis AG
Sanofi S.A.
Merck & Co., Inc.
Mitsubishi Tanabe Pharma Corporation
Cipla Ltd.
Sun Pharmaceutical Industries Ltd.
Dr. Reddy’s Laboratories Ltd.
Several leading companies have adopted robust market growth strategies in 2024. For example, Intercept Pharmaceuticals successfully deployed patient access programs across North America, increasing its market penetration in PBC drugs by 11%. AbbVie’s strategic collaboration with biotech firms to develop next-generation immunosuppressants resulted in a 9% uplift in R&D efficiency, focusing on rare autoimmune liver conditions. Takeda Pharmaceutical intensified licensing agreements in the Asia Pacific to expedite drug availability, boosting its market presence by 13% in emerging regions.
Primary Biliary Cirrhosis Drugs Market Future Outlook
Future PBC drug development will emphasize multi-targeted and combination therapies designed to modulate bile acid synthesis, inflammation, and fibrosis simultaneously. Novel agents such as selective PPAR modulators, FGF19 analogs, and immune-targeting drugs are under development, promising better tolerability and efficacy. Personalized medicine approaches, including genetic and biomarker-based treatment selection, will guide therapy optimization. Drug delivery innovations, such as sustained-release formulations, will enhance compliance, while research into regenerative liver therapies could complement pharmacological treatment in advanced disease stages.
Primary Biliary Cirrhosis Drugs Market Historical Analysis
Primary Biliary Cirrhosis drug development historically focused on symptom management through bile acid analogs like ursodeoxycholic acid (UDCA), which improved liver function and delayed disease progression. The 2010s marked a shift toward targeted therapies with the introduction of obeticholic acid, an FXR agonist that addressed disease mechanisms more effectively. Subsequent research led to the exploration of PPAR agonists and immunomodulators, expanding therapeutic options. Formulation advances improved bioavailability and patient adherence, while combination therapies began addressing treatment-resistant cases.
Sources
Primary Research interviews:
Hepatologists
Gastroenterologists
Clinical Pharmacologists
Biopharma Researchers.
Databases:
NCBI Clinical Trials
WHO Drug Database
Magazines:
Pharmaceutical Technology
PharmaTimes
BioPharma Dive
Drug Discovery & Development
Journals:
Hepatology
Liver International
Journal of Hepatology
Clinical Pharmacology & Therapeutics
Newspapers:
The Economic Times (Pharma)
The Guardian (Health)
The Wall Street Journal (Healthcare)
Business Standard (Pharmaceuticals)
Associations:
American Association for the Study of Liver Diseases (AASLD)
European Association for the Study of the Liver (EASL)
International Liver Foundation
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients