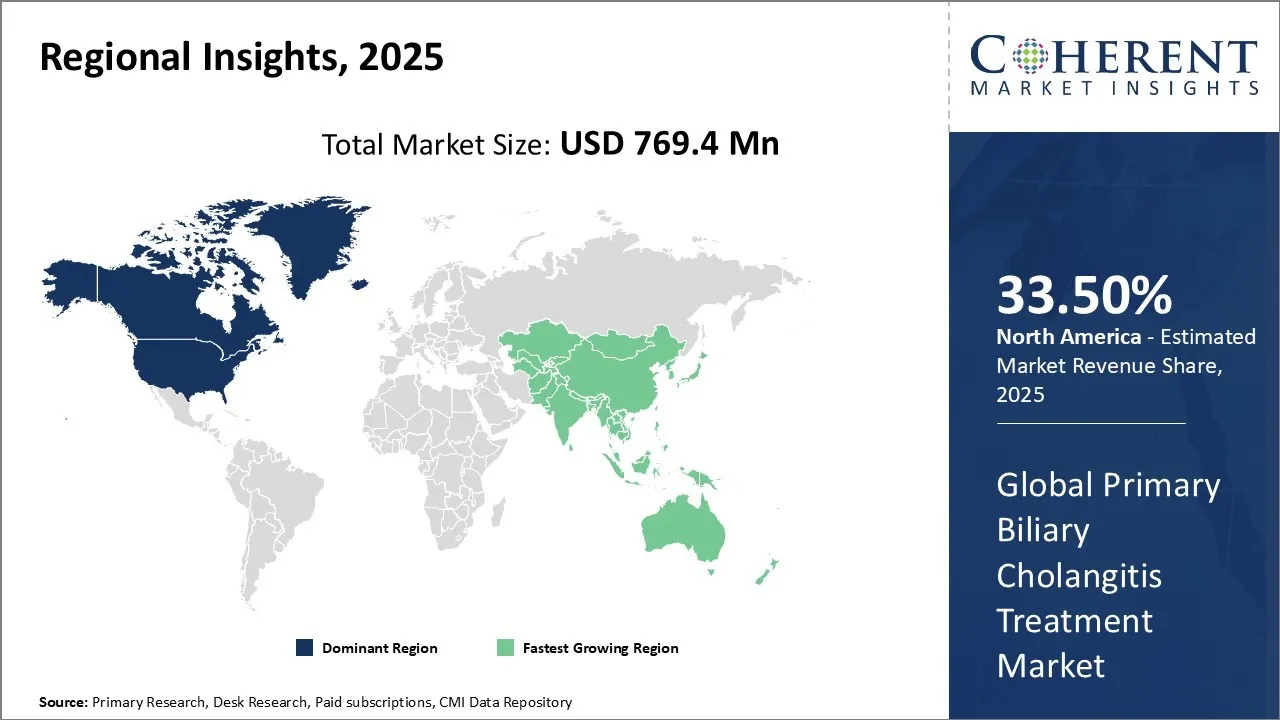
Primary Biliary Cholangitis Treatment Market size is estimated to be valued at USD 769.4 Mn in 2025 and is expected to reach USD 1,388.5 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 8.8% from 2025 to 2032.
The Primary Biliary Cholangitis Treatment Market demand is growing steadily due to rising diagnosis rates, unmet clinical needs, and new drug approvals. Increased focus on rare disease research, improved access, and support from regulatory bodies are also fueling market expansion.
Many people with PBC have no symptoms in the early stages. As the disease progresses, signs of biliary disease begin to appear. The earliest and most common symptoms of people with PBC are fatigue and itchy skin. These symptoms affect different people to different degrees. They can occur later or earlier in the course of the disease, and they can be mild to severe at any stage.
|
Current Event |
Description and its Impact |
|
FDA Regulatory Decisions on PBC Therapies |
|
|
Clinical Pipeline Developments |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Reimbursement policies for Primary Biliary Cholangitis (PBC) treatment vary significantly across global markets, shaped by healthcare system structures and drug approval status. In the United States, treatments like ursodeoxycholic acid (UDCA) and obeticholic acid (Ocaliva®) are reimbursed under Medicare and private insurance, though prior authorization and step therapy are common. In Europe, national health systems cover first-line therapies like UDCA, while second-line treatments often face delayed or conditional reimbursement. For instance, in the UK, NICE has approved elafibranor (Iqirvo®) for NHS reimbursement as a second-line option, reflecting a shift toward addressing unmet needs. Germany and France typically offer broader access under statutory insurance but may impose cost-effectiveness reviews. Japan covers PBC treatments under its national insurance scheme, though stricter inclusion criteria may apply. Emerging markets such as Brazil and India have limited public reimbursement, and patients often rely on out-of-pocket payments or private coverage. Globally, access and affordability are directly tied to health authority appraisals, with cost-effectiveness and clinical benefit being key determinants in reimbursement decisions.
In terms of treatment type, the Ursodeoxycholic acid (UDCA) segment is expected to hold largest share of the market in 2025, as it is the first line standard therapy for most PBC patients and has been widely used for decades due to its effectiveness in slowing disease progression and improving liver enzyme levels. Ursodeoxycholic acid is wide available and affordable, in comparison to Obeticholic Acid (Ocaliva) and fibrates, it also better tolerated, especially in early-stage disease.
In December 2024, the CDSCO’s Subject Expert Committee granted approval for Abbott Healthcare to manufacture and market ursodeoxycholic acid (UDCA) tablets (300 mg and 450 mg) for the treatment of obstetric cholestasis, a potentially serious liver condition during pregnancy. The decision follows comprehensive review of clinical data, and the committee has mandated a post-marketing surveillance study, with a protocol to be submitted within three months. UDCA works by improving bile flow and protecting liver cells, offering relief from severe itching and reducing risks to both mother and fetus. Abbot’s entry is expected to broaden therapeutic options in India.
In terms of distribution channel, the hospital pharmacies segment is expected to contribute the highest share of the market in 2025, owing to its ability to offer specialized prescribed medications such as ursodeoxycholic acid, obeticholic acid, which are more commonly initiated and dispensed in hospital settings. Newly approved or investigational drugs for PBC are typically distributed through hospital and specialty pharmacies due to administration protocols and monitoring needs.
Hospitals across the U.S. have begun stocking two newly FDA-approved oral drugs for Primary Biliary Cholangitis (PBC). Ipsen’s Iqirvo (elafibranor) received accelerated approval in June for use with ursodeoxycholic acid (UDCA) or as monotherapy in UDCA-intolerant patients, demonstrating reduced alkaline phosphatase and itching relief. For instance, in August 2024, Gilead’s Livdelzi (seladelpar) received its FDA clearance, showing strong biochemical responses and pruritus improvement in those with inadequate UDCA response. With inclusion in major hospital formularies, these agents offer clinicians new options for managing PBC, a rare liver disease affecting approximately 100,000 adults in the U.S.
To learn more about this report, Download Free Sample
The North America region is projected to lead the market with a 33.50% share in 2025. The region including the U.S. and Canada, has a well-established healthcare infrastructure and has a strong presence of leading key players, such as Intercept Pharmaceuticals, Inc., GSK plc., Bristol-Myers Squibb and Company, and Enanta Pharmaceuticals, focused on the research and development of primary biliary cholangitis treatment. The market for primary biliary cholangitis treatment in North America is expected to be significant, driven by the increasing research and development activities. For instance, in November 2025, Intercept Pharmaceuticals, Inc., a biopharmaceutical company, announced that results from two analyses assessing the potential of obeticholic acid (OCA) to improve outcomes for patients with primary biliary cholangitis (PBC).
Europe region is expected to exhibit the fastest growth in the market during the forecast period. Europe region has a strong focus on the development of novel therapeutics for PBC. Countries, such as Germany, France, and the U.K., have robust healthcare pharmaceutical companies, which contribute to the demand for therapeutics for PBC. The market in Europe is expected to experience steady growth due to the increasing prevalence of primary biliary cholangitis (PBC). For instance, in February 2025, the European Commission approved seladelpar (Livdelzi) as a treatment for Primary Biliary Cholangitis (PBC), complementing or replacing ursodeoxycholic acid in adults with inadequate response. The decision follows a CHMP positive opinion based on the RESPONSE trial, demonstrating improvements in alkaline phosphatase and pruritus.
The U.S. primary biliary cholangitis treatment market is characterized by the rising prevalence and early diagnosis. According to National Institute of Health, PBC affects an estimated 1 in 1,000 women over age 40 in the U.S., with rising prevalence due to better diagnostic awareness and expanded use of liver function screening tests. U.S. patients also have access to approved therapies including ursodeoxycholic acid (UDCA) and obeticholic acid (Ocaliva), which are widely available through both hospital and specialty pharmacies. Further move, strong patient advocacy from organizations like the PBCers Organization and American Liver Foundation promotes education, early testing, and support, increasing diagnosis and treatment uptake. This is further proliferating the primary biliary cholangitis market share.
The U.K. primary biliary cholangitis treatment market is rapidly expanding, fueled by its expanding treatment option for PBC including high unmet medical needs, inconsistent and substandard care and many more. Around 40% of patients don't fully respond to first-line therapy (UDCA), and over 30% fail to respond to second-line treatments. Without effective alternatives, disease progression can lead to liver failure and transplantation. National audits covering NHS hospitals have found widespread shortfalls in UDCA dosing, symptom monitoring, and transplant evaluation. PBC affects approximately 20,000–25,000 people in the UK, mainly middle-aged women, with significant impacts on quality of life and NHS costs related to transplants and complications.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 769.4 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.8% | 2032 Value Projection: | USD 1,388.5 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Intercept Pharmaceuticals, Inc., Highlight Therapeutics, S.L., GSK plc., Bristol-Myers Squibb and Company, Enanta Pharmaceuticals, NW Biotherapeutics, Merck & Co Inc., Ipsen Pharma, Johnson & Johnson, GENFIT, Ironwood Pharmaceuticals, Inc., Novartis AG, COUR Pharmaceuticals, and Kaken Pharmaceutical Co., Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The Primary Biliary Cholangitis (PBC) treatment market value is undergoing a long-overdue transformation, driven by two primary forces: a rising clinical dissatisfaction with ursodeoxycholic acid (UDCA) monotherapy and the emerging class of nuclear receptor modulators—most notably, farnesoid X receptor (FXR) agonists and peroxisome proliferator-activated receptor (PPAR) agonists.
While UDCA remains the first-line therapy, approximately 30%– 40% of patients fail to achieve biochemical response, a figure consistently reported in large cohorts such as the UK-PBC study (2016) and validated in real-world registries. The biochemical non-response population represents a clinically distinct and commercially addressable segment—one that current players like Intercept Pharmaceuticals and Genfit have rightly targeted. However, most manufacturers are failing to act with the urgency this market demands, especially given the accelerating regulatory traction in the second-line space.
Obeticholic acid (OCA), the only approved FXR agonist for PBC as of now, has shown statistically significant reductions in alkaline phosphatase (ALP) and GGT levels in Phase III POISE trials. Yet, its commercial success is increasingly constrained by two factors: pruritus-related tolerability issues and reluctance among hepatologists to adopt it as a long-term solution. This creates a clear opening for dual-acting molecules. Elafibranor, for example—a dual PPAR-α/δ agonist—has demonstrated promising interim data from the ELATIVE Phase III study, showing ALP normalization in nearly 47% of patients without the itch burden typically seen with FXR agonists.
Furthermore, the U.S. and European regulatory frameworks are beginning to favor accelerated pathways for second-line agents addressing biochemical non-responders, as evidenced by orphan drug designations and Fast Track approvals for newer entrants. These regulatory incentives, coupled with high unmet need and clear biomarker endpoints, make PBC a uniquely attractive segment for biotech investment.
In my assessment, the market is poised to pivot from mono-pathway targeting to combination regimens that modulate bile acid metabolism, fibrogenesis, and immune dysregulation concurrently. Stakeholders who invest early in dual or triple-acting molecules, especially those with differentiated safety profiles—will command not just market share but also formulary preference.
*Definition: Primary biliary cholangitis is a chronic disease in which the small bile ducts in the liver become inflamed and are eventually destroyed. When there are no bile ducts, bile builds up and causes liver damage. Over time, this damage can lead to liver scarring, cirrhosis, and eventually liver failure.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients