Pressure Relief Devices Market is estimated to be valued at USD 4,506.5 Mn in 2025 and is expected to reach USD 7,189.2 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 6.9% from 2025 to 2032. Pressure relief devices such as beds, mattresses, heel troughs, splints are used as part of the treatment to reduce or relieve the pressure on the ulcer. These devices require a power source to alternately inflate and deflate series of cells. This action has the effect of changing the areas of skin that are weight (pressure) relieved with those that are under pressure from body weight. Pressure relief devices are widely accepted for the prevention of of pressure ulcers.
Analysts’ Views on Global Pressure Relief Devices Market:
The pressure relief devices market's growth can be hindered by the increasing number of product recall. The key market players are focused on adopting organic strategies such as product launch is expected to drive the global pressure relief devices market. For instance, in March 2020, Smith and Nephew, a medical technology company, announced that they had launched the new PICO 14 Single Use Negative Pressure Wound Therapy System (sNPWT), which reduced the incidence of surgical wounds complications.
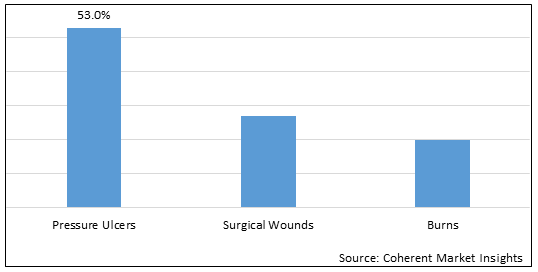
Figure 1. Global Pressure Relief Devices Market Value (%), by Application, 2025
To learn more about this report, Download Free Sample
Global Pressure Relief Devices Market- Driver
Increasing adoption of inorganic growth strategies such as acquisition by key market players is expected to drive the global pressure relief devices market over the forecast period.
Increasing adoption of inorganic growth strategies such as acquisition by key market players is expected to drive the global pressure relief devices market growth over the forecast period. For instance, in August 2022, Arjo, a medical technology company, announced that they had acquired Bruin Biometrics, LLC., a biotechnology company. Under this acquisition, Arjo will serve as an exclusive distributor for BBI’s of pressure ulcers assessment device SEM scanner that allows the early detection of pressure injury risk and thereby reduce patient suffering.
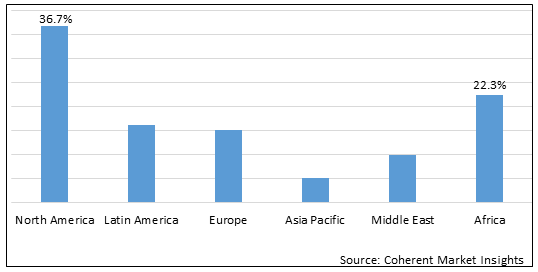
Figure 2. Global Pressure Relief Devices Market Value (US$ Million), by Region, 2025
To learn more about this report, Download Free Sample
Global Pressure Relief Devices Market- Regional Analysis
Among all regions North America is estimated to hold a dominant position in the pressure relief devices market over the forecast period. North America hold 38.4% market share due to increasing prevalence of pressure ulcers in that reagion. According to data published by the Open Nursing Journal, in Feburary 2021. The prevalence of pressure ulcers in U.S. was 38% in the systematic review of 2020
Moreover, according to the data published in National Center for Biotechnology Information, the prevalence of pressure ulcer in Africa was 18.6%
Global Pressure Relief Devices Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 can affect the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others are facing problems with the transportation of drugs from one place to another.
However, the COVID-19 pandemic had a negative impact on the global pressure relief devices market. For instance, in August 2022, according to the data published in National Center for Biotechnology Information, an observational study was performed on Eighty-seven patients which were admitted to critical care, to examine the prevalence and risk of pressure ulcers in COVID-19 patients. Hence, it was stated that 55 patients had developed pressure ulcers due to prone positioning.
Global Pressure Relief Devices Market Segmentation:
The global pressure relief devices market report is segmented into Device Type, Application, and End User
Based on Device Type, the market is segmented into Mattress, Beds, Splint, Heel Troughs, Pressure Relief Chairs. Out of which, Mattress segment is expected to dominant position in global pressure relief devices market during the forecast period and this is attributed due to increase in the usage of Mattress for the pressure relief of pain.
Based on Application, the market is segmented into an Pressure Ulcers, Surgical Wounds, Burns. Out of which, pressure ulcers segment is expected to dominate the market over the forecast period and this is attributed due to increase prevalence of pressure ulcers.
Based on End User, the heparin calcium market is segmented into hospitals, Ambulatory Surgical Centers and Clinics. Of which, hospitals segment is expected to dominate the market over the forecast period and this is attributed to the increase in number of hospitals.
Among all segmentation, application segment has highest potential due to increasing pressure ulcer over the forecast period. For instance, in October 2020, according to the data published in National Center for Biotechnology Information, it was stated that about 2.5 million hospitalizations in the U.S. in 2019 are due to pressure ulcers which is a major concern for patients and healthcare staff.
Global Pressure Relief Devices Market Cross Sectional Analysis:
In application segment, pressure ulcer is dominant in North America region due to increasing prevalence of pressure ulcer. According to the data published by University of Huddersfield in November 2021, A cross sectional survey was conducted in England to explore the prevalence of pressure ulcer. Hence, the overall prevalence of pressure ulcer was 9.04%.
Global Pressure Relief Devices Market: Key Developments
Increasing adoption of inorganic growth strategies such as product launch by key market players is expected to drive the global pressure relief devices market over the forecast period.
Pressure Relief Devices Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 4,506.5 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.9% | 2032 Value Projection: | USD 7,189.2 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Medtronic, Arjo, Hill-Rom Holdings, Inc., Invacare Corporation, Smith & Nephew Plc., Stryker, Talley Group Ltd., Paramount Bed Co., Ltd., wissner-bosserhoff GmbH, 3M, 5 Minds Mobility |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Pressure Relief Devices Market: Key Trends
Increasing prevalence of pressure ulcers in intensive care units is expected to drive the global pressure relief devices market over the forecast period.
Increasing prevalence of pressure ulcers in intensive care units is expected to drive the global pressure relief devices market over the forecast period. In February 2022, according to the data published in Dove Press Medical Journal, A study was conducted among 125 patients treated at the Department of Anesthesiology of the 4th Military Teaching Hospital in Wroclaw between 2019 and 2020 to check the prevalence of pressure ulcers in intensive care unit patients. Hence, it was estimated that the prevalence of pressure ulcer was 8.8%.
Global Pressure Relief Devices Market: Restraint
Increasing number of product recalls by regulatory authorities such as the U.S. Food and Drug Administration is expected to hinder the pressure relief devices market over the forecast period.
Increasing number of product recalls by regulatory authorities such as the U.S. Food and Drug Administration is expected to hinder the pressure relief devices market over the forecast period. For instance, in June 2022, the U.S. Food and Drug Administration recalled a class II device MRS surface with X-ray mattress, which was manufactured by Hill-Rom Holdings, Inc., a medical technology company. The device was recalled because the failure mode on the affected mattresses can cause a reduction in the performance of the microclimate management feature used to pull heat and moisture away from the patient resulting in an increased patient risk of pressure ulcer development.
Global Pressure Relief Devices Market- Key Players
Major players operating in the global pressure relief devices market include Medtronic, Arjo, Hill-Rom Holdings, Inc., Invacare Corporation, Smith & Nephew Plc., Stryker, Talley Group Ltd., Paramount Bed Co., Ltd., wissner-bosserhoff GmbH, 3M, 5 Minds Mobility
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients