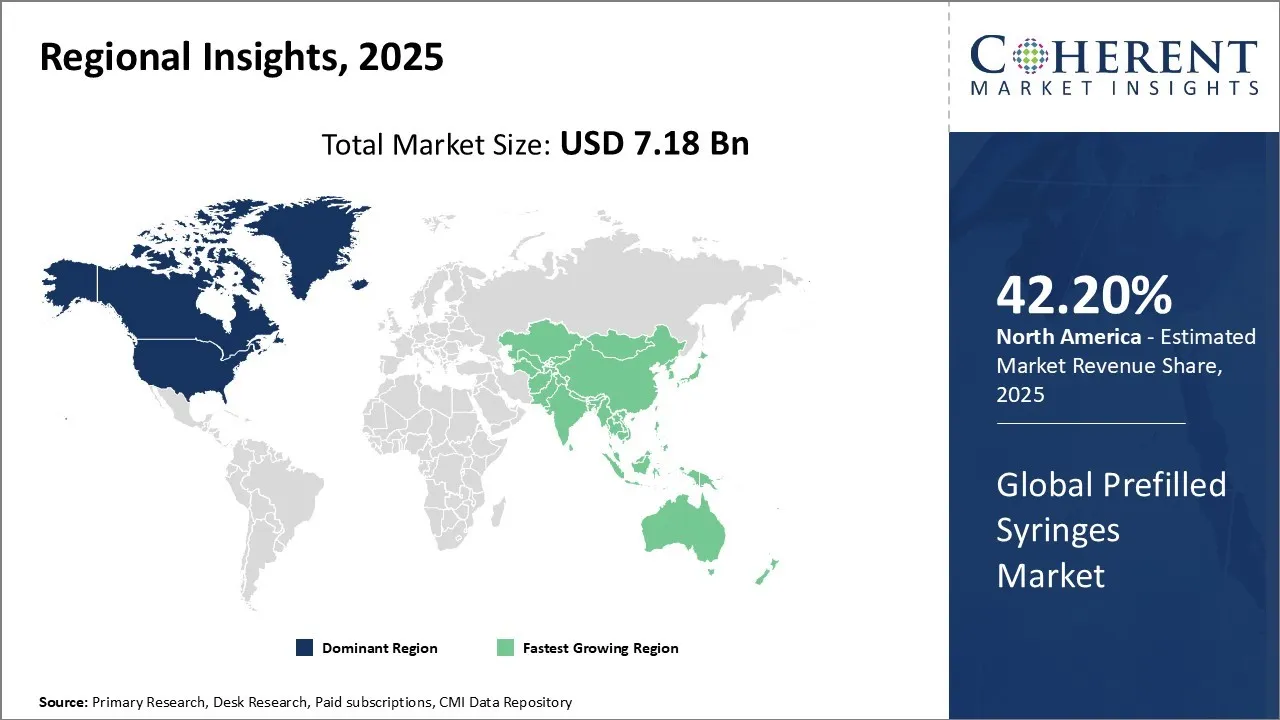
Prefilled Syringes Market is estimated to be valued at USD 7.18 Bn in 2025 and is expected to reach USD 10.51 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of5.6% from 2025 to 2032.
The Global Prefilled Syringes Market demand is rising due to increasing adoption in chronic disease management, vaccine delivery, and biologics. Enhanced patient safety, reduced dosing errors, and improved convenience are driving healthcare providers toward prefilled formats. Technological advancements in syringe materials and manufacturing processes further support market expansion across hospitals, clinics, and home care settings.
|
Current Event |
Description and its Impact |
|
Regulatory Framework Evolution and Compliance Changes |
|
|
Technological Innovation and Market Transformation |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
In terms of drug type, the biologics segment is expected to capture 38.5% share in 2025, driven by rising demand for monoclonal antibodies, therapeutic proteins, and blood products. These complex drugs require precise dosing and sterile delivery, making prefilled syringes ideal. Their use in chronic conditions like cancer and autoimmune diseases boosts adoption across global healthcare systems.
For instance, in June 2025, Biocon Biologics received Health Canada approval for Yesafili™ (aflibercept), marking a key milestone in ophthalmology biosimilars. The company announced its first global launch is scheduled for July 2025, positioning Yesafili™ as a cost-effective alternative for treating retinal diseases and expanding access to advanced biologic therapies in Canada.
In terms of material, the glass segment is expected to hold the largest share of the market in 2025, due to their excellent chemical stability, drug compatibility, and barrier properties. They are preferred for sensitive biologics and high-value formulations, minimizing interaction risks and preserving drug efficacy. Despite the rise of polymers, glass remains the gold standard in hospital and specialty care settings.
For instance, in September 2024, BD announced a major expansion of its manufacturing capacity for advanced prefillable syringes, aiming to support next-generation biologics. The initiative includes enhancements to the injection experience and increased production capabilities across global sites, reinforcing BD’s commitment to innovation and meeting rising demand in the Prefilled Syringes Market.
In terms of design, the single-chamber segment is projected to lead with the highest share of the market in 2025, due to their simplicity, cost-effectiveness, and widespread use in routine injections. They offer ready-to-use convenience, reduce preparation time, and lower contamination risk. Their compatibility with a broad range of drugs makes them the preferred design in both clinical and home care environments.
For instance, in March 2024, Otsuka Pharmaceutical announced the launch of Abilify® Prefilled Syringe in Japan, offering a new administration format for its antipsychotic treatment. Designed for intramuscular injection, the prefilled syringe enhances convenience and safety for healthcare providers, supporting improved adherence and therapeutic outcomes in patients with schizophrenia and bipolar disorder.
In terms of product type, the staked in-needle prefilled syringes segment expected to have the greatest share of the market in 2025, favored for their integrated design, which eliminates assembly steps and enhances sterility. This format improves patient safety and reduces dosing errors, especially in high-throughput hospital settings. Their ergonomic design also supports ease of use for healthcare professionals and patients alike.
In terms of usability, the disposable segment is expected to account for the maximum share of the market in 2025, due to infection control, convenience, and regulatory compliance. They eliminate the need for sterilization, reduce cross-contamination risks, and support single-use protocols in hospitals and clinics. Their growing use in vaccination and chronic disease management further drives demand.
For instance, in July 2025, the FDA approved a single-dose prefilled syringe format of Shingrix, GSK’s shingles vaccine, aimed at simplifying administration and improving efficiency. The new presentation eliminates the need for reconstitution, enhancing convenience for healthcare providers and supporting broader immunization efforts across clinical settings.
In terms of manufacturing method, the captive segment is projected to dominate with the biggest share of the market in 2025, leads as pharmaceutical companies increasingly produce prefilled syringes in-house to maintain quality control, ensure regulatory compliance, and protect proprietary formulations. This approach supports scalability, reduces outsourcing risks, and aligns with strategic goals for supply chain resilience and innovation.
In terms of end user, the hospital segment is expected to lead the market with a colossal share in 2025, driven by high patient volumes, complex drug regimens, and the need for efficient medication delivery. Prefilled syringes streamline workflows, reduce preparation time, and enhance safety in acute care settings. Their use in emergency, surgical, and chronic care departments reinforces dominance.
For instance, in August 2025, Accord BioPharma announced the U.S. commercial launch of Imuldosa™ (ustekinumab-srlf) prefilled syringes, a biosimilar to Stelara®. Offered at the lowest wholesale acquisition cost (WAC) among branded ustekinumab biosimilars, Imuldosa™ aims to improve access and affordability for patients requiring treatment for plaque psoriasis and psoriatic arthritis.
To learn more about this report, Download Free Sample
In 2025, North America remains the dominant region with 42.2% share in the Global Prefilled Syringes Market, driven by advanced healthcare infrastructure, widespread adoption of biologics, and strong regulatory frameworks supporting injectable drug delivery systems. The region benefits from high patient awareness and demand for safety-enhanced, ready-to-use formats.
For instance, in August 2025, BD announced a $35 million investment to expand its Nebraska facility, boosting production of prefilled flush syringes. The move aims to strengthen U.S. healthcare supply chains, enhance patient safety, and meet rising demand for ready-to-use syringe formats in hospitals and clinical settings nationwide.
Asia-Pacific emerges as the fastest growing region, fueled by expanding healthcare access, rising chronic disease prevalence, and increased investment in pharmaceutical manufacturing. Countries like China, India, and Japan are witnessing rapid uptake of prefilled syringes due to their efficiency, sterility, and suitability for large-scale immunization and chronic care programs.
For instance, in August 2025, SCHOTT announced record-breaking performance in its primary pharmaceutical packaging segment, driven by strong global demand for prefillable glass syringes and vials. The company expanded production capacity and emphasized innovation in drug containment solutions, reinforcing its leadership in supplying high-quality packaging for biologics and injectable therapies worldwide.
In 2025, the United States leads global demand in the Prefilled Syringes Market, fueled by high biologics consumption and a robust healthcare infrastructure. The widespread adoption of self-injection devices for chronic conditions like diabetes and rheumatoid arthritis further accelerates growth. Regulatory support and patient preference for convenience and safety also drive market expansion.
For instance, in July 2025, GSK announced that the U.S. FDA approved Shingrix in a prefilled syringe presentation. This new format simplifies vaccine administration by eliminating reconstitution steps, enhancing efficiency for healthcare providers. The single-dose prefilled syringe supports broader immunization efforts against shingles, especially in high-volume clinical and pharmacy settings.
In 2025, Germany stands out as a key European market in the Prefilled Syringes sector, backed by its robust pharmaceutical manufacturing capabilities and high standards in hospital care. The country’s emphasis on precision drug delivery and safety drives widespread adoption of prefilled syringes, especially for biologics and chronic therapies in clinical and inpatient settings.
For instance, in August 2025, SCHOTT Pharma launched a ready-to-use polymer cartridge designed for wearable drug delivery devices. The innovation supports biologics and high-viscosity formulations, offering enhanced patient comfort and convenience. This advancement aligns with growing demand for self-administration solutions and reinforces SCHOTT’s commitment to next-generation pharmaceutical packaging technologies.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 7.18 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5.6% | 2032 Value Projection: | USD 10.51 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Gerresheimer AG, Becton, Dickinson and Company, West Pharmaceutical Services, Inc., Terumo Corporation, Nipro Europe Group Companies, B. Braun SE, Sanofi, GSK plc., Pfizer Inc., Bayer AG, Polymedicure, Amsino International, Inc., Leeford Healthcare Limited, Torrent Pharmaceuticals Ltd., Regeneron Pharmaceuticals Inc., Ypsomed AG, SOL-Millennium, Stevanato Group S.p.A. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Increasing product launches by market players are significantly contributing to the expansion of the Prefilled Syringes Market size. Leading pharmaceutical and biotech companies are introducing innovative syringe formats tailored for biologics, vaccines, and chronic therapies. These launches focus on improving drug stability, patient convenience, and safety features, thereby accelerating adoption across hospitals, clinics, and home care settings. Enhanced usability, integrated needle systems, and compatibility with sensitive formulations are driving competitive differentiation and fueling global market growth.
The increasing prevalence of chronic diseases and infections is a major driver of Prefilled Syringes Market growth in 2025. Conditions such as diabetes, rheumatoid arthritis, and infectious diseases require frequent and precise drug administration. Prefilled syringes offer a safe, convenient, and ready-to-use solution, reducing dosing errors and improving patient compliance, especially in outpatient and home care settings.
*Definition: A prefilled syringe is a single-dose container of medication or a vaccination, to which the manufacturer has attached a needle. Pre-filled syringes give improved dosage accuracy, require fewer steps for injection, and pose less danger of harm and cross-contamination. Pre-filled syringes are frequently taken into consideration as early as Phase II in the drug development process and are now a generally acknowledged component of the medication life-cycle. Prefilled syringe cartridges are made to fit into particular syringes that are used to give different liquid drugs. These replace traditional syringes, which healthcare professionals must manually fill before each dose is given. Prefilled syringe cartridges provide the benefits of simplicity, low cost, precision, sterility, and safety.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients