Preclinical assets is a fully integrated CRO (Clinical Research Organization) providing services to support drug discovery programs from target discovery through IND (Investigational New Drug) filing and managing Phase I-IV clinical trials. Preclinical resources, services for HTS (High-throughput screening) and assay development, synthetic organic and medicinal chemistry, DMPK/in-vivo pharmacology and safety pharmacology, toxicology, as well as clinical trial services for the regulatory approval of novel drug and medical device products.
Global preclinical assets market size is valued at US$ 5,250.2 million in 2022 and is expected to witness a CAGR of 7.5% over the forecast period (2022 – 2030).
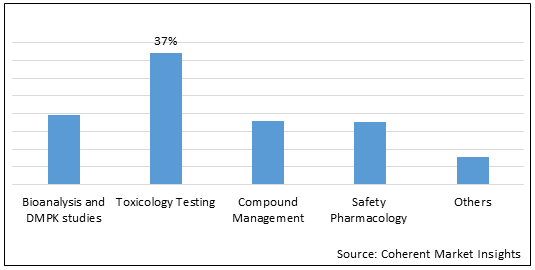
Figure 1.Global Preclinical Assets Market Share (%), by Service, 2022
To learn more about this report, Download Free Sample
Global Preclinical Assets Market– Drivers
Increasing number of new test launch in the toxicology by the market players is expected to drive growth of the global preclinical assets market over the forecast period.
Increasing number of launch of new toxicology test by the key market players is expected to drive growth of the global preclinical assets market over the forecast period. For instance, in October 2018, SGS SA, a multinational company announced the launch of the in-vitro testing which consists of the development of present cell/tissue culture capabilities, flow cytometry, and mass spectrometry facilities, along with the introduction of high throughput screening, automation, and multiplexing technologies. The launch of this toxicology test will help the company to further strengthen and expand its product portfolio.
Preclinical Assets Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: | US$ 5,250.2 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 7.5% | 2030 Value Projection: | US$ 9,778.8 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Eurofins Scientific, ICON plc, WuXi AppTec, Viroclinics Xplore, Medpace, Inc., Charles River Laboratories., Pharmatest Services, PPD Inc., SGS SA (SGS), Intertek Group plc, Labcorp Drug Development, Laboratory Corporation of America, Inc., Crown Bioscience, Comparative Biosciences, Inc., TCG Lifesciences Private Limited., Shanghai Medicilon Inc., Domainex, Absorption Systems, AmplifyBio, and IQVIA |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
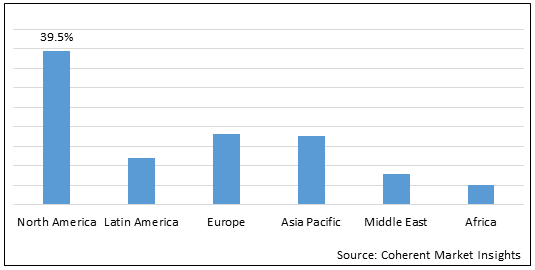
Figure 2. Global Preclinical Assets Market Share (%), by Region, 2022
To learn more about this report, Download Free Sample
Increasing number of inorganic strategies such as acquisition by market players in North America is expected to drive growth of the global preclinical assets market over the forecast period.
Inorganic strategies such as acquisition by the key market players in North America are expected to drive growth of the global preclinical assets market over the forecast period. For instance, on May 9, 2022, Labcorp, a global life sciences company, and AtlantiCare, a health care organization in southern New Jersey, U.S., announced that they had closed a transaction to expand their long-term strategic relationship. Labcorp will acquire select assets from AtlantiCare’s clinical outreach business, which serves the AtlantiCare Physician Group and Affiliated Physicians and their patients across southern New Jersey, U.S. This collaboration will help the company in further expansion of laboratory services.
Global Preclinical Assets Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe and the World Health Organization had declared it a public health emergency on January 30, 2020.
The sudden outbreak of COVID-19 has bought the world to a standstill. The whole world is fighting this pandemic with increased burden on hospital and healthcare professionals. However this has also opened new opportunities in the digital health platforms. The need for virtual consulting is anticipated to rise significantly during the current economic crisis.
Increasing number of inorganic strategies by the key market players during covid-19 pandemic, was expected to bolster growth of the global preclinical assets during the pandemic. For instance, in December 2021, Viroclinics-DDL, a global Contract Research Organization and CEPI, the Coalition for Epidemic Preparedness Innovations, signed an agreement which allowed Viroclinics-DDL to produce and distribute laboratory stocks of the Omicron SARS-CoV-2 variant for use in CEPI’s centralized COVID-19 vaccine testing network. The produced Omicron SARS-COV-2 variant was used for the performance of laboratory assays, assessing the neutralizing potential of COVID-19 vaccines against the new variant (Omicron).
Global Preclinical Assets Market: Key Developments
In September 2021, Viroclinics-DDL, a global contract research organization, announced the launch of Viroclinics Xellerate, a newly formed Business Unit focused on clinical trial support and global logistics services.
On February 17, 2022, Viroclinics-DDL, a global contract research organization, announced that Cerba Research, the group’s global clinical trial central and specialty laboratory services division, had merged with Viroclinics-DDL, a global specialist virology and immunology contract research organization (CRO). This merger will help the company in further expanding and transforming diagnostic solutions for clinical trials
In November 2019, Envigo RMS LLC, a global supplier of research models and associated services, announced an agreement to acquire the assets of the research models business unit of Horizon Discovery Group plc., a research company. This acquisition will help the company in further expansion of the services in clinical research.
Global Preclinical Assets Market: Restraint
One of the major restrain in the global preclinical assets market is lack of standardization as per international regulatory requirements such as:
Key Players
Key players operating in the global preclinical assets market include Eurofins Scientific, ICON plc, WuXi AppTec, Viroclinics Xplore, Medpace, Inc., Charles River Laboratories., Pharmatest Services, PPD Inc., SGS SA (SGS), Intertek Group plc, Labcorp Drug Development, Laboratory Corporation of America, Inc., Crown Bioscience, Comparative Biosciences, Inc., TCG Lifesciences Private Limited., Shanghai Medicilon Inc., Domainex, Absorption Systems, AmplifyBio, and IQVIA
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients