Postoperative Pain Management Market Size and Forecast – 2026 – 2033
The global postoperative pain management market is valued at about USD 41.2 billion in 2026 and is forecast to grow to approximately USD 65.36 billion by 2033, expanding at a CAGR of around 5.4 % from 2026‑2033.
Global Postoperative Pain Management Market Overview
Postoperative pain management involves strategies to reduce pain following surgery, aiming to improve recovery, mobility, and overall patient outcomes. It includes pharmacological approaches such as opioids, nonsteroidal anti-inflammatory drugs (NSAIDs), local anesthetics, and adjuvant medications, as well as non-pharmacological methods like physiotherapy, nerve stimulation, and psychological support. Effective pain management minimizes complications, shortens hospital stays, and enhances patient satisfaction. With increasing surgical procedures worldwide and growing awareness of opioid-related risks, multimodal analgesia—combining different therapies—is becoming standard. The market is expanding due to advancements in drug delivery systems, minimally invasive techniques, and personalized pain management approaches.
Key Takeaways
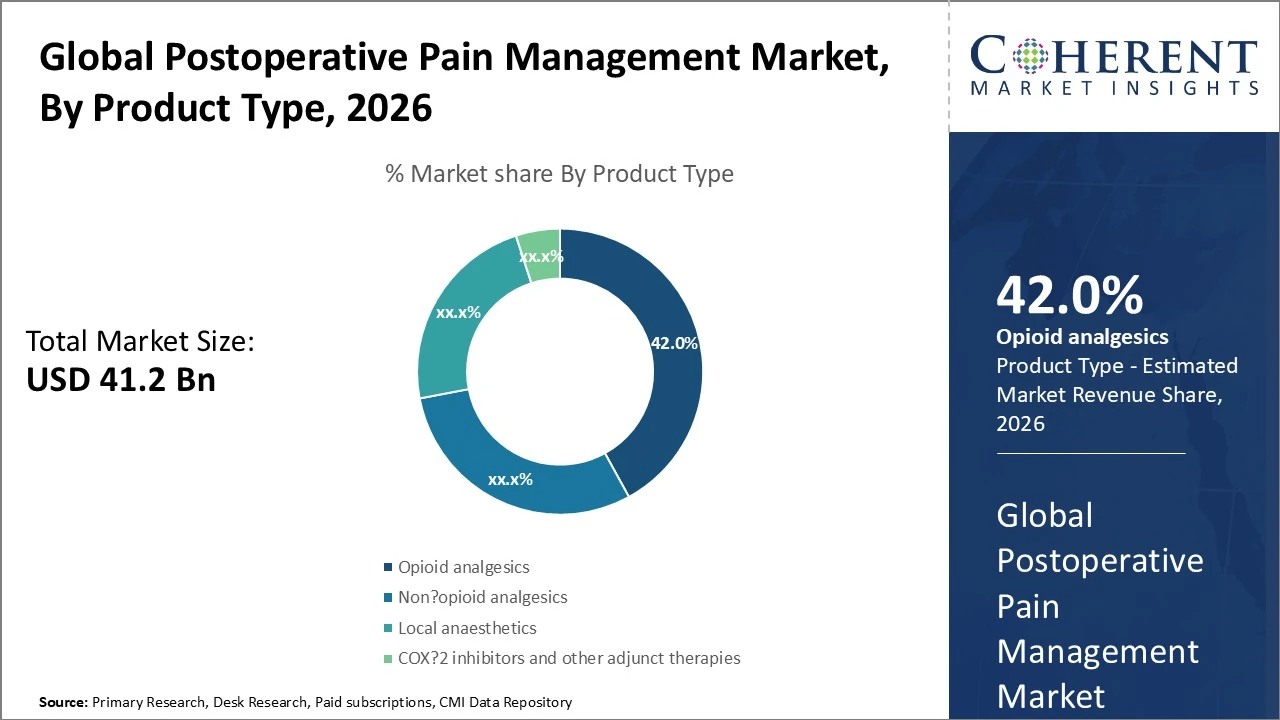
Product Type- Opioid analgesics remain the largest segment, holding about 42 % of market share.
Patient‑controlled analgesia remains the largest technology segment, representing roughly 50% of technology‑based revenue.
Hospitals dominate the postoperative pain management market, accounting for about 65 % of total revenue.
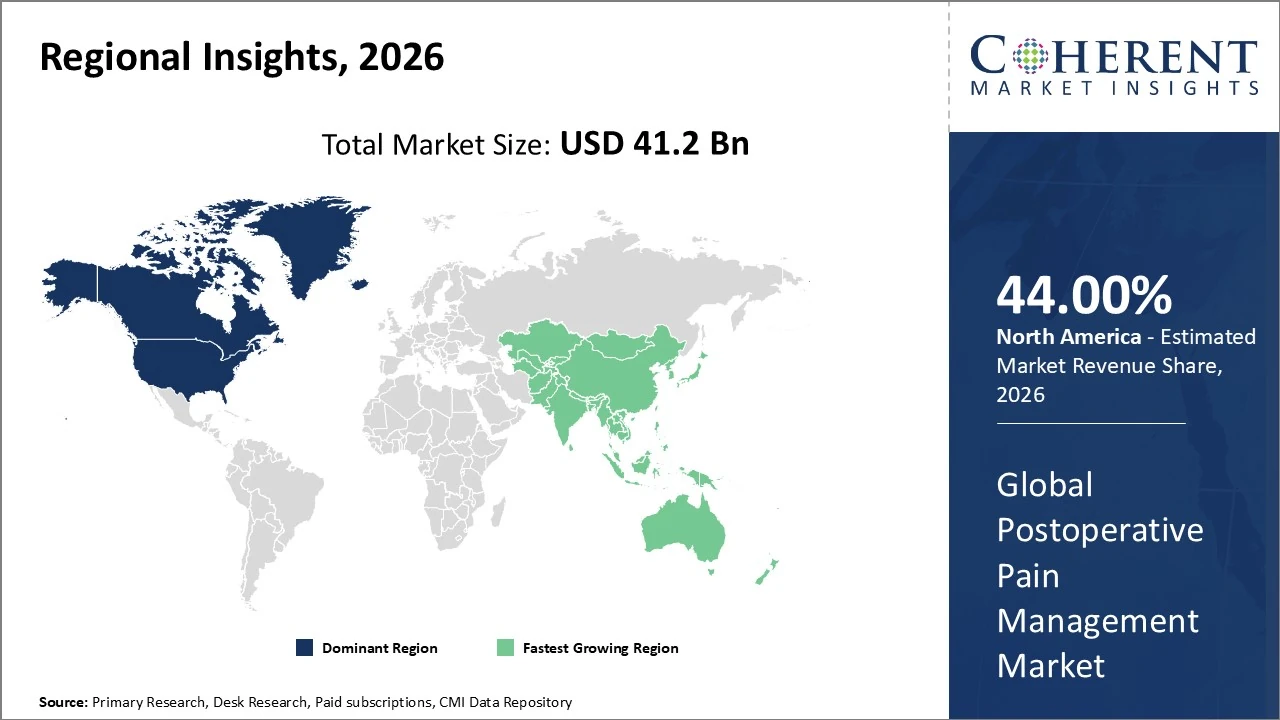
In North America, the postoperative pain management market holds the largest regional share, around 44 % of global revenue.
In Asia Pacific, the postoperative pain management market is one of the fastest‑growing regional segments, projected to account for around 28.7 % of the global market.
United States comprises about 87% of the North American postoperative pain management market.
Postoperative Pain Management Market Segmentation Analysis
To learn more about this report, Download Free Sample
Postoperative Pain Management Market Insights, By Product Type
The postoperative pain management market is segmented by product type into opioid analgesics, non‑opioid analgesics/NSAIDs, and local anaesthetics. Opioid analgesics remain the largest segment, holding about 42 % of market share due to their effectiveness in managing moderate to severe surgical pain. Non‑opioid analgesics/NSAIDs make up around 30 % of the market, driven by safety concerns and opioid‑sparing trends. Local anaesthetics are rapidly growing, capturing around 23 % as opioid‑sparing multimodal approaches gain popularity. COX‑2 inhibitors and other adjunct therapies contribute the remaining share of 5%. Growth is driven by advances in formulations and reduced opioid dependency protocols.
Postoperative Pain Management Market Insights, By Technology
The postoperative pain management market by technology is segmented into patient‑controlled analgesia (PCA), long‑acting analgesics, and nerve blocks. Patient‑controlled analgesia remains the largest technology segment due to personalized dosing and strong adoption in hospitals, representing roughly ~50% of technology‑based revenue. Long‑acting analgesics—including extended‑release formulations hold about ~30% as demand grows for prolonged pain relief. Nerve blocks and regional techniques account for around ~20%, driven by rising use of targeted, opioid‑sparing pain control approaches post‑surgery. Ongoing innovation in these technologies supports broader use and improved recovery outcomes.
Postoperative Pain Management Market Insights, By End-User
Hospitals dominate the postoperative pain management market, accounting for about 65 % of total revenue due to high volumes of inpatient surgical procedures and comprehensive pain control infrastructure. Ambulatory Surgical Centers (ASCs) hold around 23 %, driven by the growth of outpatient surgeries and minimally invasive techniques that require effective, rapid pain relief. Home care settings and specialty clinics collectively represent roughly 12 %, reflecting expanding early discharge protocols, telehealth support, and patient‑centered recovery models outside traditional hospital environments. These trends underscore the shift toward decentralized care while hospitals remain central to complex postoperative pain management services.
Postoperative Pain Management Market Trends
Increasing adoption of combinations of opioids, non-opioids, and local anesthetics to reduce opioid dependence and improve recovery outcomes.
Rising outpatient procedures and minimally invasive surgeries are driving demand for effective, fast-acting, and patient-controlled pain management solutions.
Innovations in drug delivery systems, long-acting formulations, patient-controlled analgesia devices, and nerve block techniques are enhancing personalized, targeted postoperative pain control.
Postoperative Pain Management Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Postoperative Pain Management Market Analysis and Trends
In North America, the postoperative pain management market holds the largest regional share, around 44 % of global revenue, led by the United States with extensive surgical volumes and advanced healthcare infrastructure. Key trends include a shift toward multimodal and opioid‑sparing pain protocols, driven by regulatory and clinical emphasis on safety and recovery outcomes; strong R&D and FDA approvals for innovative analgesics; and integration of digital health tools for pain monitoring. The region is forecast to grow steadily, supported by high healthcare expenditure and expanding outpatient procedures.
Asia Pacific Postoperative Pain Management Market Analysis and Trends
In Asia Pacific, the postoperative pain management market is one of the fastest‑growing regional segments, projected to account for around 28.7 % of the global market due to expanding surgical volumes and healthcare investments in China, India, Japan and South Korea. Growth is driven by rising awareness of effective pain control, increasing adoption of multimodal analgesia and long‑acting formulations, and improving healthcare infrastructure. The region’s CAGR is ~6.5 % through 2032, with demographic shifts, higher surgical demand, and opioid‑sparing strategies further accelerating market penetration.
Postoperative Pain Management Market Outlook for Key Countries
USA Postoperative Pain Management Market Analysis and Trends
In the United States, which comprises about 87% of the North American postoperative pain management market, demand is driven by high surgical volumes across orthopedic, cardiac, and laparoscopic procedures. The market is shifting from traditional opioids toward multimodal analgesia, including non‑opioid and regional anaesthesia options, supported by FDA approvals of novel agents like OLINVYK and extended‑release formulations. Hospitals and ambulatory surgery centers lead usage due to emphasis on Enhanced Recovery After Surgery (ERAS) protocols and opioid‑sparing strategies. Growth is supported by advanced healthcare infrastructure, strong R&D pipelines, and regulatory focus on safer pain control, with consistent year‑on‑year adoption increases.
Germany Postoperative Pain Management Market Analysis and Trends
In Germany, the postoperative pain management market is a leading force in Europe, representing approximately 26.9 % of Europe’s market share due to high surgical volumes and advanced clinical practices. German hospitals widely adopt multimodal analgesia and Enhanced Recovery After Surgery (ERAS) protocols, emphasizing non‑opioid and regional pain control approaches. The nation’s universal healthcare system and strong reimbursement support accelerate uptake of innovative analgesics and personalized pain management strategies. Growing awareness of opioid‑sparing therapies further influences prescribing behaviours. Continued investment in pain‑management technologies and evidence‑based care is expected to sustain stable market growth.
Analyst Opinion
Analysts highlight that increasing numbers of orthopedic, cardiac, and minimally invasive surgeries are fueling the need for effective postoperative pain control.
The market is moving away from opioid-only treatments toward combinations of NSAIDs, local anesthetics, and adjuvants to improve safety and recovery.
Devices such as patient-controlled analgesia (PCA) systems, long-acting formulations, and regional anesthesia techniques are enhancing personalization and market adoption.
Analysts see Asia Pacific and Latin America as high-growth regions due to rising surgical rates, healthcare investment, and awareness.
Stricter opioid regulations and growing emphasis on evidence-based protocols are accelerating R&D and adoption of safer alternatives.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 41.2 Billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 5.4% | 2033 Value Projection: | USD 65.36 Billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Pfizer, Johnson & Johnson, AbbVie, Teva Pharmaceuticals, Mylan, Hikma Pharmaceuticals | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Postoperative Pain Management Market Growth Factors
The postoperative pain management market is growing due to several key factors. Rising surgical procedure volumes, particularly in orthopedics, cardiology, and minimally invasive surgeries, increase demand for effective pain control. The shift toward multimodal analgesia and opioid-sparing protocols drives adoption of non-opioid and regional therapies. Technological advancements in patient-controlled analgesia devices, long-acting formulations, and nerve blocks enhance treatment efficacy. Growing awareness among patients and healthcare providers about postoperative recovery and pain management improves market penetration. Additionally, expanding healthcare infrastructure in emerging markets, coupled with favorable reimbursement policies and R&D investment, further accelerates market growth globally.
Postoperative Pain Management Market Development
In January 2025, Vertex Pharmaceuticals Incorporated announced that the U.S. Food and Drug Administration (FDA) approved JOURNAVX™ (suzetrigine), an oral, non-opioid, highly selective NaV1.8 pain signal inhibitor, for the treatment of adults with moderate-to-severe acute pain.
Key Players
Leading Companies of the Market
Pfizer Inc.
Johnson & Johnson
AbbVie
Teva Pharmaceuticals
Mylan
Hikma Pharmaceuticals
Leading companies in the postoperative pain management market include Pfizer, Johnson & Johnson, AbbVie, Mylan, Teva Pharmaceuticals, Hikma Pharmaceuticals. These firms drive market growth through innovative analgesics, patient-controlled analgesia devices, multimodal therapies, and regional anesthesia solutions, leveraging strong R&D and global distribution networks.
Postoperative Pain Management Market Future Outlook
The future outlook of the postoperative pain management market is highly positive, driven by rising surgical volumes, increasing demand for faster recovery, and growing awareness of effective pain control. The shift toward multimodal analgesia and opioid-sparing strategies will continue to reshape treatment protocols. Technological innovations, including long-acting analgesics, patient-controlled analgesia devices, and regional anesthesia techniques, will enhance personalized care. Expansion in emerging markets, coupled with improved healthcare infrastructure and favorable reimbursement policies, will further accelerate growth. Additionally, integration of digital health solutions for pain monitoring and management is expected to improve outcomes, efficiency, and patient satisfaction globally.
Postoperative Pain Management Market Historical Analysis
The postoperative pain management market has historically experienced steady growth due to increasing surgical procedures worldwide and rising awareness of effective pain control. Initially dominated by opioid analgesics, the market saw gradual adoption of NSAIDs, local anesthetics, and regional anesthesia techniques to reduce opioid-related risks. Hospitals and surgical centers have traditionally driven demand, with patient-controlled analgesia (PCA) devices emerging in the early 2000s. Regulatory emphasis on safety, coupled with clinical guidelines promoting multimodal pain management, influenced market evolution. Over the past decade, investments in long-acting formulations, minimally invasive surgery adoption, and enhanced recovery protocols have shaped the market’s growth trajectory.
Sources
Primary Research Interviews:
Healthcare Providers
Hospital Administrators
Pharmaceutical & Biotech Executives
Medical Device Professionals
Databases:
PubMed / Medline
ClinicalTrials.gov
WHO Global Health Observatory
Journals:
The Journal of Pain
Anesthesiology
Regional Anesthesia and Pain Medicine
British Journal of Anaesthesia
Newspapers:
The Wall Street Journal
Financial Times
The Guardian
The New York Times
Associations:
American Society of Anesthesiologists (ASA)
American Pain Society (APS)
International Association for the Study of Pain (IASP)
European Society of Anesthesiology and Intensive Care (ESAIC)
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients