Post-traumatic stress disorder (PTSD) is a mental health condition that gets activated by a traumatic event either experienced or witnessed such as natural disaster, serious accident, sexual violence, a terrorist act, etc. PTSD can occur in people of all ages. Some of the major symptoms of post-traumatic stress disorder are intrusive memories, avoidance, negative changes in thinking and mood, as well as changes in emotional and physical reactions. The common drugs prescribed by doctors for PTSD are antidepressants, anti-anxiety medications, fluoxetine, and others.
Global Post-traumatic Stress Disorder Treatment Market is estimated to be valued at US$ 1034.3 million in 2022 and is expected to witness a CAGR of 5.1% over the forecast period (2022 – 2030).
Figure 1. Global Post-traumatic Stress Disorder Treatment Market Share (%), by Drug Class, 2022
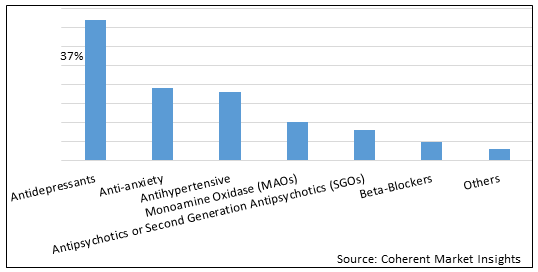
To learn more about this report, Download Free Sample
Global Post-traumatic Stress Disorder Treatment Market– Drivers
Increasing number of inorganic strategies such as product launches, collaborations, expansion, and partnerships to develop drugs for the treatment of post-traumatic stress disorder is expected to drive the growth of the global post-traumatic stress disorder treatment market over the forecast period.
Increasing number of product launch and collaboration between the key market players in the treatment of PTSD is expected to drive the growth of the global post-traumatic stress disorder treatment market over the forecast period. For instance, on March 30, 2022, Sun Pharmaceutical Industries Ltd., a pharmaceutical company, entered into a patent licensing agreement with H. Lundbeck, a pharmaceutical company, to market and distribute its own version of Vortioxetine (an antidepressant pill) in India under the brand name Vortidiftm. Vortioxetine is a novel antidepressant with multimodal activity, which is approved to treat Major Depressive Disorder (MDD) in adults. The product is approved in more than 80 countries, including the U.S., Europe, Canada, and Australia.
Figure 2. Global Post-traumatic Stress Disorder Treatment Market Share (%), by Region, 2022
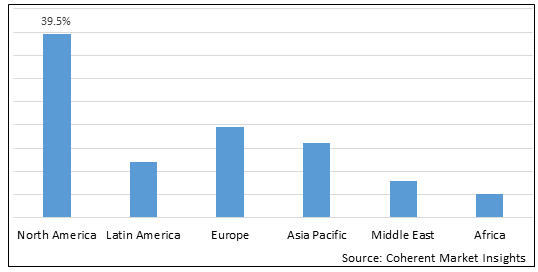
To learn more about this report, Download Free Sample
Increasing number of research and development for the treatment of post-traumatic stress disorder by market players in North America is expected to drive the growth of the global post-traumatic stress disorder treatment market over the forecast period.
Incrasing research and development by the key market players in the treatment of post-traumatic stress disorder are expected to boost the global post-traumatic stress disorder treatment market over the forecast period. For instance, in December 2020, Tonix Pharmaceuticals Holding Corp., a pharmaceutical company, announced positive results from its Phase 3 RECOVERY study1 of TNX-102 SL 5.6 mg, for the treatment of civilian and military-related post-traumatic stress disorder.
Global Post-traumatic Stress Disorder Treatment Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe and the World Health Organization declared it a public health emergency on January 30, 2020.
The sudden outbreak of COVID-19 has bought the world to a standstill. The whole world is fighting this pandemic with an increased burden on hospitals and healthcare professionals. However, this has also opened new opportunities in digital health platforms. The need for virtual consulting is anticipated to rise significantly during the current economic crisis.
During the pandemic, the demand for the treatment of post-trauma stress disorder increased. According to the PTSD Foundation of America, in 2022, the COVID-19 infection and post-traumatic stress disorder do not have a direct connection but, the norms to stop the spread of the virus from person to person could be a potential trigger for post-traumatic stress disorder. The norms made by the WHO to stop the spread of COVID infection included maintaining social distancing and quarantining patients if they were found COVID-19 positive. These may have led to a gradual increase in the number of patients with post-trauma stress disorder. For instance, on January 4, 2022, according to an article published by National Center for Biotechnology Center, U.S. National Library of Medicine, there were approximately 16.8% of incidence of PTSD symptoms in nurses of China during Covid-19 pandemic.
Post-traumatic Stress Disorder Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: | US$ 1034.3 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 5.1% | 2030 Value Projection: | US$ 1536.8 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Jazz Pharmaceuticals plc., Madrigal Mental Care, Allergan PLC, Otsuka Pharmaceutical Co., Ltd., apex laboratories Pvt. Ltd., H. Lundbeck A/S (Lundbeck), Neurovation Labs, Inc., Eli Lilly and Company, GlaxoSmithKline PLC, Sun Pharmaceuticals Pvt Ltd, Bristol-Myers Squibb, Johnson and Johnson, Pfizer Inc., Aurobindo Inc., Amneal Pharmaceuticals LLC, Teva Pharmaceuticals Industries Ltd., And Mylan Pharmaceuticals (Viatris Inc.) |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Post-traumatic Stress Disorder Treatment Market: Key Developments
In May 2020, Allied Corp., a pharmaceutical company, announced the signing of a manufacturing and distribution agreement with MGC Pharmaceuticals, a pharmaceutical company. According to the agreement, MGC Pharmaceuticals will manufacture Allied Corp.’s products. MGC currently has cannabis-based pharmaceutical products for sale in Europe and has demonstrated a proven ability to commercialize these products. Commercialization of Allied’s post-traumatic stress disorder (PTSD) treatment products into the European, Latin America, and North American marketplace is a key strategy of Allied’s
On November 4, 2019, Bionomics limited, a global clinical-stage biopharmaceutical company, announced that the U.S. Food and Drug Administration (FDA) granted Fast Track designation to the BNC210 development program for the treatment of post-traumatic stress disorder (PTSD) and other trauma-related and stressor-related disorders.
In October 2020, Jazz Pharmaceuticals plc, a pharmaceutical company, and SpringWorks Therapeutics, Inc., a biotechnology company, announced that Jazz Pharmaceuticals Ireland Limited and SpringWorks entered into an asset purchase and exclusive license agreement under which Jazz had acquired SpringWorks' Fatty Acid Amide Hydrolase ("FAAH") inhibitor program including PF-04457845 in US$ 375 million. Jazz will initially focus on developing PF-04457845 for the potential treatment of PTSD and associated symptoms. PF-04457845 represents an innovative approach to treating PTSD with a novel mechanism of action that has the potential to address multiple core symptoms of the disease, including fear extinction, anxiety, and disrupted sleep architecture.
Global Post-traumatic Stress Disorder Treatment Market: Restraint
One of the major restraints of the global post-traumatic stress disorder treatment market is lack of awareness and side effects associated with the drugs, such as Sertraline, Paroxetine, and others used in the treatment of post-traumatic stress disorder.
The primary side effects of sertraline include syncope, lightheadedness, diarrhea, nausea, sweating, dizziness, xerostomia, confusion, hallucinations, tremor, somnolence, impotency, fatigue, rhinitis, and female sexual disorder. Also, there is a bleeding risk associated with sertraline, as it may inhibit platelet aggregation. Whereas, the side effects associated with paroxetine include changes in mood, anxiety or behavior, serotonin syndrome or neuroleptic malignant syndrome-like reactions, eye problems, severe allergic reactions, bruising/ bleeding, seizures or convulsions, manic episodes, changes in appetite or weight, low sodium levels, and bone fracture.
Key Players
Key players operating in the Global Post-traumatic Stress Disorder Treatment Market include Jazz Pharmaceuticals plc., Madrigal Mental Care, Allergan PLC, Otsuka Pharmaceutical Co., Ltd., apex laboratories Pvt. Ltd., H. Lundbeck A/S (Lundbeck), Neurovation Labs, Inc., Eli Lilly and Company, GlaxoSmithKline PLC, Sun Pharmaceuticals Pvt Ltd, Bristol-Myers Squibb, Johnson and Johnson, Pfizer Inc., Aurobindo Inc., Amneal Pharmaceuticals LLC, Teva Pharmaceuticals Industries Ltd., and Mylan Pharmaceuticals (Viatris Inc.)
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients