Polycythemia vera is a rare, chronic disorder involving the overproduction of blood cells in the bone marrow (myeloproliferation). The overproduction of red blood cells is most changing, but the production of white blood cells and platelets are also elevated in most cases. Since red blood cells are overproduced in the marrow, this leads to abnormally high numbers of circulating red blood cells within the blood. Consequently, the blood thickens and increases in volume, a condition called hyperviscosity. Thickened blood may not flow through smaller blood vessels properly. A variety of symptoms can occur in individuals with polycythemia vera including nonspecific symptoms such as headaches, fatigue, weakness, dizziness or itchy skin, an enlarged spleen (splenomegaly), a variety of gastrointestinal issues and the risk of blood clot formation, which may prevent blood flow to vital organs such as heart, lungs, kidneys.
The global polycythemia vera therapeutics market is estimated to be valued at US$ 1,115.9 million in 2021 and is expected to exhibit a CAGR of 4.5% over the forecast period (2021-2028).
Figure 1. Global Polycythemia Vera Therapeutics Market Share (%), By Region, 2021
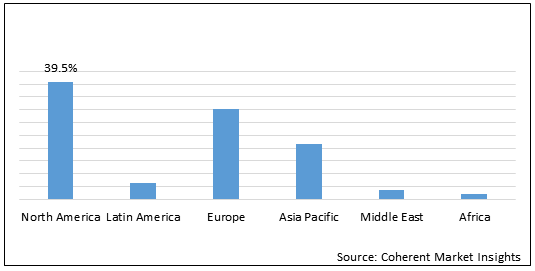
To learn more about this report, Download Free Sample
The increasing incidence of polycythemia vera is expected to drive the growth of the global polycythemia vera therapeutics market over the forecast period.
For instance, according to the article in journal of Multidisciplinary Digital Publishing Institute (MDPI) in July 2020, the estimated annual incidence rates for polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis are 0.84, 1.03, and 0.47 per 100,000 population globally and the prevalence is much higher, particularly for PV and ET, as mortality rates are relatively low globally.
Furthermore, according to the National Organization for Rare Disorders, around 44 to 57 per 100,000 people in the U.S. are suffering from polycythemia vera.
Moreover, symptoms of polycythemia vera usually develop slowly over many years in patients. Often, the disorder is found incidentally on a blood test, as part of a routine exam before noticeable symptoms occur. Occasionally, affected individuals may report vague, nonspecific symptoms that eventually lead to diagnosis of the disorder. Several individuals with polycythemia vera slowly develop a variety of general, nonspecific symptoms that are common to many disorders such as headaches, fatigue, weakness, dizziness, excessive sweating especially at night, and itchy skin that, in severe cases, may be worse after taking a shower or a warm bath.
Polycythemia Vera Therapeutics Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2020 | Market Size in 2019: | US$ 1,115.9 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2022 to 2028 |
| Forecast Period 2021 to 2028 CAGR: | 4.5% | 2028 Value Projection: | US$ 1,516.5 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Pfizer Inc., Galena Biopharma, Bristol-Myers Squibb Company, Novartis AG, Eli Lilly and Company, PharmaEssentia Corporation, Bayer AG, Mylan N.V., Teva Pharmaceuticals Industries Ltd, GlaxosmithKline plc, ANP Technologies, INC., F. Hoffmann-La Roche Ltd., Gilead Sciences, Inc, Karus Therapeutics Limited, and Miragen Therapeutics, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Figure 2. Global Polycythemia Vera Therapeutics Market Value (US$ Mn), By Drug Class, 2021
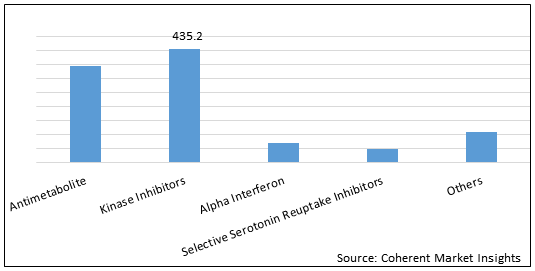
To learn more about this report, Download Free Sample
The increasing research and development activities for the development of novel therapeutics for the treatment of polycythemia vera is expected to drive the market growth over the forecast period.
Key players operating in the market are focusing on submitting application for approval of drugs therapy for the treatment of polycythemia vera, which is expected to drive growth of the global polycythemia vera therapeutics market over the forecast period.
For instance, in May 2021, PharmaEssentia USA Corporation, a subsidiary of Taiwan-based PharmaEssentia Corp., a global biopharmaceutical innovator company, announced the resubmission of its Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA), seeking approval for ropeginterferon alfa-2b-njft for the treatment of polycythemia vera (PV). The resubmission follows receipt of a complete response letter in March 2021, in which the U.S. FDA sought additional information regarding the administration format with the product. Importantly, no concerns were raised regarding the clinical profile of the product. Morever, noted were COVID-related restrictions that delayed the required pre-approval inspection of the company’s manufacturing facility in Taiwan.
Ropeginterferon alfa-2b-njft has orphan drug designation for the treatment of PV in the U.S. is marketed as Besremi in Europe. The product was approved by the European Medicines Agency (EMA) in 2019. The Ropeginterferon alfa-2b-njft molecule was invented and is manufactured by PharmaEssentia.
Furthermore, on June 3, 2021, Protagonist Therapeutics, a biopharmaceutical company, announced that the U.S. Food and Drug Administration (FDA) has granted Breakthrough Therapy Designation for its lead investigational new drug candidate, rusfertide, for the treatment of patients with polycythemia vera (PV) for the reduction of erythrocytosis in those patients, who do not require further treatment for thrombocytosis and/or leukocytosis.
Global Polycythemia Vera Therapeutics Market – Impact of Coronavirus (Covid-19) Pandemic
During this Covid-19 pandemic, pharmaceutical business, clinical tool companies and likewise biotechnology firms are facing difficulties that are occurring from the interruption in supply chains and the demand to transform service processes. As the researches reveal that there is no relationship in between blood cancer cells as well as coronavirus, hence polycythemia vera therapy market is being affected by the pandemic. If Covid-19 pandemic proceeds for a medium/long duration, it may impact the supply of product and also active ingredients around the world along with the import as well as export of drugs. There has been a disturbance in the treatment market. As coronavirus has actually ended up being the major emphasis on polycythemia vera therapeutics market and all study institutes, biotech as well as pharmaceutical companies are selected in cooperation job to manage Covid-19, who are previously engaged in research and development of therapeutics for the treatment of polycythemia vera.
Global Polycythemia Vera Therapeutics Market Restraint
The strict pricing and reimbursement policies, very high cost of drugs used in the treatment of polycythemia vera are the factors that are expected to hinder growth of the global polycythemia vera therapeutics market over the forecast period.
Key Players
Major players operating in the global polycythemia vera therapeutics market include Pfizer Inc., Galena Biopharma, Bristol-Myers Squibb Company, Novartis AG, Eli Lilly and Company, PharmaEssentia Corporation, Bayer AG, Mylan N.V., Teva Pharmaceuticals Industries Ltd, GlaxosmithKline plc, ANP Technologies, INC., F. Hoffmann-La Roche Ltd., Gilead Sciences, Inc, Karus Therapeutics Limited, and Miragen Therapeutics, Inc.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients