Point of Care Infectious Disease Diagnostics Market - Insights
Point of care diagnosis involves testing of the samples at the point of care to facilitate rapid diagnosis and decide on the treatment plan accordingly. Infectious diseases often need to be diagnosed early, as they can turn lethal against immunity system. Point of care diagnosis allows for early detection of diseases and can greatly impact the medical outcome of the disease treatment. Advanced technologies used in point-of-care diagnostics such as lateral flow, agglutination assays, and solid phase are integrated with innovative features such as portability of handheld devices. The U.S. Food & Drug Administration (FDA) is responsible for overseeing all the activities associated with marketing of point of care diagnostic products. Along with FDA, the Clinical Laboratory Improvement Amendments (CLIA) also regulates these products. Point of care tests are efficient and save time and costs due to various benefits provided by it such as preventing sample spillage, no necessity of skilled professionals, as the tests can be performed easily, and low costs. Some of the commercially available point of care diagnostics include CoaguChek Pro II, Uni-Gold HIV, Accu-Chek Aviva meter, Alere I Influenza A & B, OneTouch Verio Flex, and ICON SC Strep A.
Advances in technology of point of care diagnostics is expected to support the growth of the point of care infectious disease diagnostics market
Centralized laboratory testing is the gold standard and has established in biochemistry, hematology, and diagnostics. Point-of-care diagnostic offers various advantages such as ease of use in remote areas, technological advancements, and lowered price points. Small handheld point-of-care devices are portable and can be used by patients themselves. These tests use fingerstick or capillary samples, which are directly applied to instrument and avoid need of sample containers. Technologies such as lateral flow, various molecular assays integrated with point-of-care have changed the outlook of point-of-care diagnostics. For instance, products offered by leading manufacturers such as Alere and Roche are immunoassays, which are cost-effective and provide rapid results. Furthermore, in 2014, Becton, Dickinson, and Company offered point-of-care system BD FACSPresto for HIV/AIDS. It offers percentage results of CD4 T lymphocytes and hemoglobin concentration. Moreover, Infectious Disease Research Institute (IDRI) developed a fusion antigen in January 2017, which is used as a part of diagnostic test manufactured by InBios International, Inc. for Chagas disease.
The global point of care infectious disease diagnostics market was valued at US$ 1,764.6 million in 2016 and is expected to witness a robust CAGR of 14.5% over the forecast period (2017 – 2025).
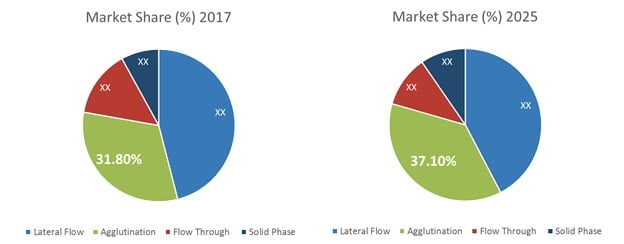
Figure 1. Global Point of Care Infectious Disease Diagnostics Market Share, by Technology (%) 2017 and 2025
To learn more about this report, Download Free Sample
Initiatives and funding from individual organizations for point-of-care infectious disease diagnostics is expected to boost the market growth over the forecast period
Various organizations and governments are focused on research and development for infectious diseases to provide effective diagnostic and treatment options. For instance, QuantuMDx Group received funding from Bill & Melinda Gates Foundation to develop and test company’s CAPTURE-XT pathogen concentration technology and Q-POC molecular diagnostic platform for rapid low cost detection of tuberculosis in 2016. In January 2018, University of Glasgow-led project received US$ 1.85 million funding, which is a part of Global Challenges Research Fund (GCRF). This project will develop new tests for parasitic diseases and point-of-care testing in remote locations to help enable rapid diagnosis, and rapid treatment.
However, limitations associated with point-of-care testing such as specific steps and timing and different methodologies than laboratory testing, which may provide difference in the test results from laboratory testing may hinder the growth of the market. Moreover, in remote locations proper inventory system must be planned before to prevent deterioration, lack of stock, and shelf life issues which may further hinder growth of the market.
Major players operating in the point of care infectious disease diagnostics market include Danaher Corporation, Abbott Laboratories, Alcon Laboratories, Inc., Hoffman-La Roche Ltd., Becton, Dickinson, and Company, Trinity Biotech Plc, AccuBioTech Co., Ltd. and Cardinal Health, Inc.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients