Pneumonia Testing Market Size and Forecast – 2025 – 2032
The Global Pneumonia Testing Market size is estimated to be valued at USD 3.48 billion in 2025 and is expected to reach USD 6.47 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 9.3% from 2025 to 2032.
Global Pneumonia Testing Market Overview
Pneumonia testing kits and devices include molecular diagnostic assays, rapid antigen tests, multiplex PCR panels, and biomarker-based systems. Products such as cartridge-based PCR analyzers and lateral-flow antigen kits provide quick, point-of-care detection of bacterial or viral pneumonia. Laboratory systems offer high-throughput respiratory panels capable of identifying multiple pathogens simultaneously. Biomarker assays (procalcitonin, CRP) help distinguish bacterial from viral infections. Modern pneumonia testing products emphasize faster turnaround, higher sensitivity, and integration with digital reporting systems for hospitals and clinics.
Key Takeaways
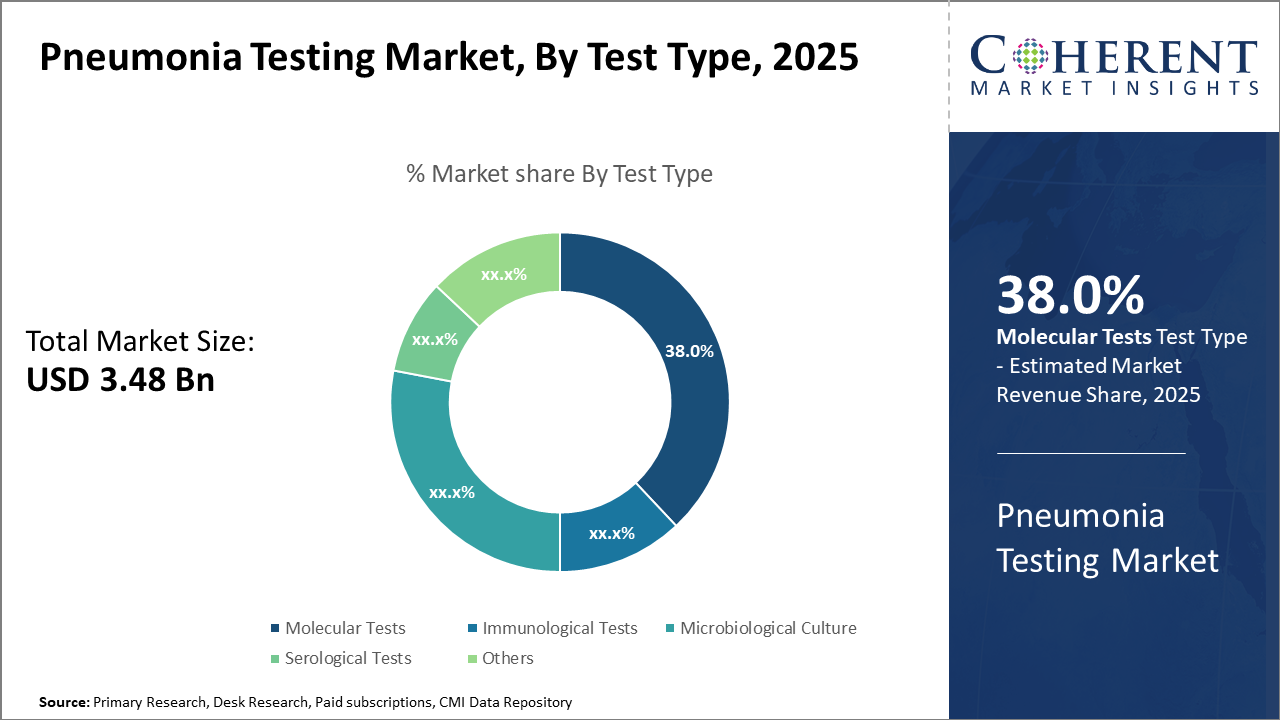
Molecular Tests dominate with a 38% industry share driven by diagnostic accuracy and speed, while Immunological Tests show the fastest growth due to cost-effectiveness and simplicity.
End User segment analysis highlights hospitals as the largest revenue contributor amid rising inpatient pneumonia cases; home care testing is growing rapidly with increasing outpatient care.
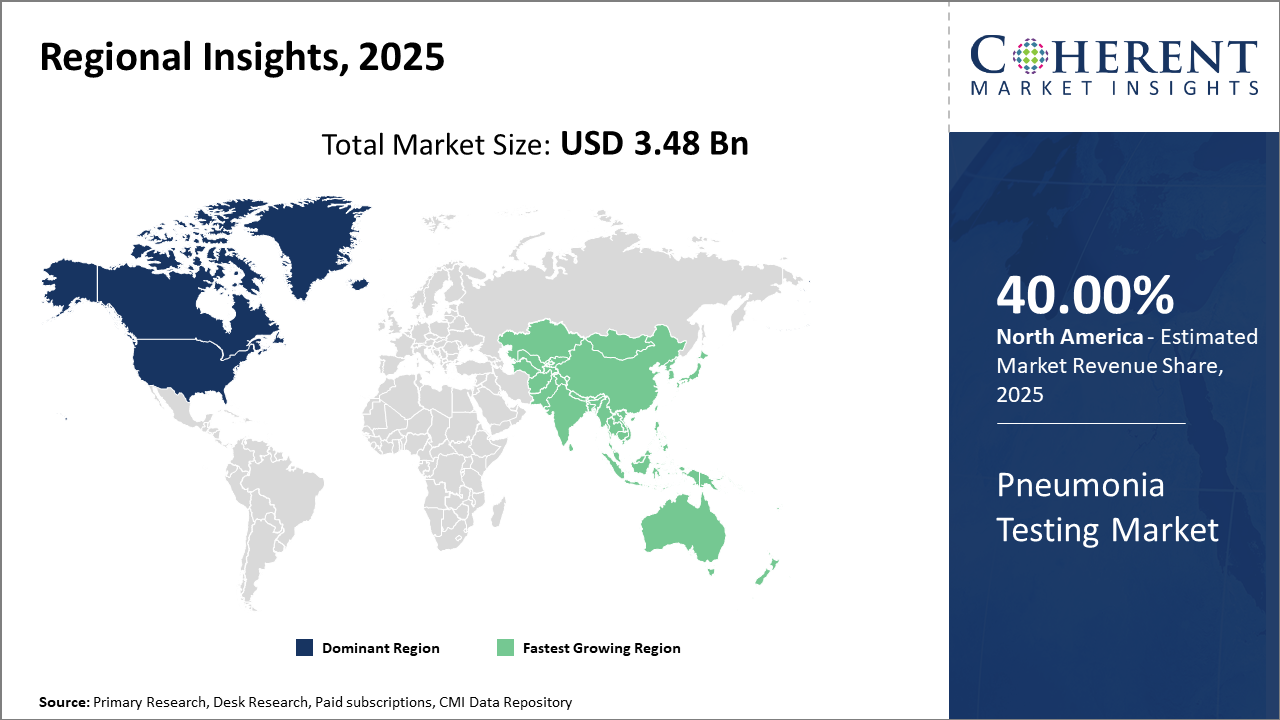
From a regional perspective, North America commands the largest market share with 40% due to advanced healthcare infrastructure, whereas the Asia Pacific exhibits the fastest CAGR owing to expanding healthcare capacity and rising pneumonia incidences.
Pneumonia Testing Market Segmentation Analysis
To learn more about this report, Download Free Sample
Pneumonia Testing Market Insights, By Test Type
Molecular Tests, comprising nearly 38% of the industry share, lead due to their high sensitivity and rapid turnaround time. These tests largely utilize polymerase chain reaction (PCR) technology to detect pneumonia-causing pathogens with precision, thus supporting early intervention and improving clinical outcomes. Their expanding application in multiplex panels that identify multiple variants simultaneously drives further penetration. The increasing prevalence of molecular diagnostics aligns with rising pneumonia incidences and the demand for actionable clinical data. Immunological Tests represent the fastest-growing subsegment, recognized for their relatively low cost and ease of use. Employing antibody detection mechanisms like ELISA, these tests cater to widespread screening programs and resource-limited settings.
Pneumonia Testing Market Insights, By End User
Hospitals maintain the largest industry share due to heavy inpatient pneumonia caseloads necessitating comprehensive diagnostic workflows. This segment benefits from fully equipped labs and the capability to adopt advanced diagnostic technologies rapidly. Diagnostic Laboratories serve as specialized testing hubs supporting hospitals and external healthcare providers, driven by increasing outsourcing trends and centralized high-throughput testing demands.
Pneumonia Testing Market Insights, By Technology
PCR technology dominates due to its unparalleled sensitivity and specificity, enabling the identification of even low pathogen loads in clinical samples, which is essential for accurate pneumonia diagnosis. Its adaptability to multiplex formats further strengthens its position and accelerates adoption across clinical settings. ELISA, popular for immunological testing, benefits from comparatively low operational complexity and cost-effectiveness, supporting broad-based screening initiatives.
Pneumonia Testing Market Trends
The Pneumonia Testing Market trends reveal an accelerated pivot towards multiplex polymerase chain reaction (PCR) technologies that enable simultaneous detection of bacterial and viral etiologies.
For instance, multiple healthcare facilities across Europe adopted next-generation sequencing in 2024, providing precise pathogen identification and influencing treatment protocols.
Additionally, demand for rapid point-of-care testing has surged sharply, evidenced by over 20% annual growth in lateral flow assay kits in North American outpatient centers.
Market players are also leveraging AI-powered platforms to enhance predictive accuracy and reduce diagnostic errors, which is reshaping the testing ecosystem.
Pneumonia Testing Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Pneumonia Testing Market Analysis and Trends
In North America, the dominance in the Pneumonia Testing Market is reinforced by robust healthcare infrastructure, high patient awareness, and proactive government investments in diagnostic technology development. Companies headquartered in North America benefit from strong R&D capabilities and well-established reimbursement frameworks, promoting continuous market expansion.
Asia Pacific Pneumonia Testing Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth with a CAGR exceeding 11%, primarily fueled by increasing pneumonia incidence, expanding diagnostic labs, and government programs enhancing disease detection capabilities in countries like India and China. Trade dynamics and healthcare modernization initiatives have facilitated an influx of innovative pneumonia testing products, catering to the extensive and diverse population base. Local manufacturing capacities enhancement and growing collaborations between the public and private sectors further accelerate this regional development.
Pneumonia Testing Market Outlook for Key Countries
USA Pneumonia Testing Market Analysis and Trends
The United States leads the Pneumonia Testing Market with extensive adoption of next-gen molecular diagnostics and rapid point-of-care testing formats. In 2024, the U.S. FDA approved several multiplex PCR assays improving pneumonia pathogen detection accuracy, directly impacting market revenue. Strategic collaborations between diagnostic companies and major hospital networks have expedited test availability, contributing to aggressive market growth. The presence of renowned companies like Roche Diagnostics and Abbott Laboratories entrenches the country’s market leadership.
India Pneumonia Testing Market Analysis and Trends
India represents a rapidly evolving pneumonia diagnostic landscape characterized by rising government health initiatives to improve respiratory disease management. Public health bodies increased procurements of molecular and serological testing kits by over 35% in 2025. Additionally, expanding healthcare infrastructure and growing awareness among rural populations are significant market growth facilitators. Partnerships with global test manufacturers have improved access to advanced diagnostic solutions, creating opportunities for both international and domestic market players.
Analyst Opinion
Increasing demand for rapid diagnostic tests has become a key quantitative driver for the pneumonia testing market revenue. For example, the adoption of point-of-care molecular tests in emergency departments surged by approximately 27% in 2024, reducing turnaround time to under an hour, which significantly improves patient triage. This demand enhances market dynamics by shifting focus from traditional culture-based methods towards nucleic acid amplification tests.
Supply chain optimization has notably contributed to enhanced production capacities across diagnostic kit manufacturers in 2024. Several manufacturers increased output by nearly 15%, responding to surges in pneumonia testing during recent global respiratory illness outbreaks. This supply-side advancement underpins upward market momentum and strengthens the industry share in key regions such as North America and Europe.
Expanding pneumonia testing applications in pediatric and geriatric care have propelled market growth strategies, with pediatric cases accounting for over 35% of test volume in 2025, as per hospital records. The use of multiplex testing panels allows differential diagnosis amid overlapping respiratory symptoms, directly impacting market size by broadening use cases.
Rising healthcare infrastructure investments in the Asia Pacific have translated into increased imports of advanced pneumonia testing kits. For instance, India witnessed a 40% increase in imports of molecular diagnostic equipment in early 2025, reflecting heightened market revenue streams in emerging regions. This demand-side indicator forecasts substantial market expansion opportunities.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 3.48 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.3% | 2032 Value Projection: | USD 6.47 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Abbott Laboratories, Roche Diagnostics, BioMérieux SA, Thermo Fisher Scientific, Becton Dickinson and Company, Cepheid (Danaher Corporation), BD Diagnostics, DiaSorin S.p.A., Sysmex Corporation, Ortho Clinical Diagnostics. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Pneumonia Testing Market Growth Factors
The shift towards rapid and precise molecular diagnostics governs the pace of market growth, with innovation cycles becoming shorter and more dynamic, as seen by the release of novel multiplex assays in 2024. Additionally, increased pneumonia-related hospitalization globally has intensified test demand, especially during seasonal infection surges. Government funding programs aimed at combating lower respiratory tract infections have expanded testing infrastructure in emerging economies, pushing market scope forward. Moreover, the growing threat of antibiotic resistance has reinforced the need for swift, accurate diagnostics, enabling targeted therapies and reducing unnecessary antibiotic use.
Pneumonia Testing Market Development
In January 2025, CARB-X awarded $1 million to Rhode Island Hospital at Brown University Health to develop a PCR-based method, guided by RNA sequencing, for detecting bacterial pneumonia directly from whole blood.
In August 2025, UK-based EDX Medical developed a test to detect and characterise early pneumonia in critically ill patients and partnered with Cambridge University Hospitals NHS Foundation Trust’s ICU to advance its development.
Key Players
Leading companies of the market:
Abbott Laboratories
Roche Diagnostics
BioMérieux SA
Thermo Fisher Scientific
Becton Dickinson and Company
Cepheid (Danaher Corporation)
BD Diagnostics
DiaSorin S.p.A.
Sysmex Corporation
Ortho Clinical Diagnostics
Competitive strategies reveal a focus on partnerships and technological enhancements: For instance, a prominent PCR test manufacturer entered a strategic collaboration with governmental health bodies in 2024, which increased test deployment by 30% across multiple regions. Similarly, the introduction of AI-powered diagnostic platforms by some key players has improved detection accuracy by 15%, translating into enhanced market share and revenue.
Pneumonia Testing Market Future Outlook
In the future, pneumonia testing will continue moving toward faster, decentralized, and more accurate solutions. Growth will be fueled by the need for antimicrobial stewardship and differentiation between bacterial and viral infections. AI-assisted imaging and portable molecular devices will become standard in both hospital and primary-care settings. Integration of testing platforms with electronic medical records will enhance disease tracking and reporting. Additionally, cost-effective molecular assays and telehealth-linked diagnostics will expand access in developing regions, supporting early detection and better clinical management of respiratory diseases.
Pneumonia Testing Market Historical Analysis
The pneumonia testing market has progressed from traditional culture-based methods to rapid molecular diagnostics over the past two decades. Initially, testing relied heavily on chest X-rays and microbiological assays that required several days for pathogen identification. The 2010s marked a turning point with the introduction of multiplex PCR panels capable of detecting multiple respiratory pathogens simultaneously. The COVID-19 pandemic significantly accelerated the adoption of advanced molecular and antigen-based assays, leading to expanded diagnostic infrastructure and awareness of respiratory disease surveillance.
Sources
Primary Research Interviews:
Pulmonologists
Microbiologists
Clinical Pathologists
Diagnostic Laboratory Directors
Infectious Disease Specialists
Databases:
WHO Global Health Observatory
ClinicalTrials.gov
GlobalData Diagnostics Reports
CDC Respiratory Disease Data
Magazines:
Diagnostics World
Medical Laboratory Observer
Clinical Lab Products
MedTech Insight
Journals:
Journal of Clinical Microbiology
The Lancet Respiratory Medicine
Diagnostic Microbiology and Infectious Disease
Chest Journal
Newspapers:
The Washington Post (Health)
The Guardian (Science)
The Hindu (Health)
The Wall Street Journal (Medical)
Associations:
World Health Organization (WHO)
Infectious Diseases Society of America (IDSA)
American Thoracic Society (ATS)
European Society of Clinical Microbiology and Infectious Diseases (ESCMID)
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients