Plexiform neurofibroma is the most common peripheral nerve sheath tumor of the orbit involving multiple nerve bundles, causing periorbital and orbital soft-tissue hypertrophy, primary or compensatory bony changes, and ocular abnormalities. Neurofibromatosis is a genetic disorder. It is inherited in an autosomal dominant pattern with variable penetrance. Plexiform neurofibromas develop during early childhood, often before the cutaneous neurofibromas have fully developed. The tumors in these disorders are usually noncancerous (benign), but sometimes can become cancerous (malignant).
The global plexiform neurofibromas treatment market is estimated to be valued at US$ 1,371.14 million in 2022 and is expected to exhibit a CAGR of 8.2% during the forecast period (2022-2030).
Figure 1. Global Plexiform Neurofibromas Treatment Market Share (%), by Drug Class, 2022
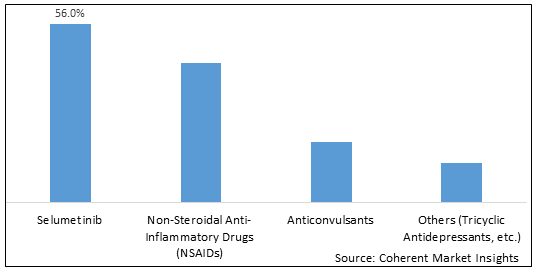
To learn more about this report, Download Free Sample
Global Plexiform Neurofibromas Treatment Market - Driver
Increasing adoption of inorganic strategies such as product approval by regulatory authorities such as Japanese Ministry of Health, Labour and Welfare (MHLW), is expected to drive the global plexiform neurofibromas treatment market over the forecast period. For instance, in September 2022, AstraZeneca, a biopharmaceutical company, announced that the Japanese Ministry of Health, Labour and Welfare (MHLW), approved Koselugo (selumetinib) in Japan for the treatment of plexiform neurofibromas with clinical symptoms, such as pain and disfigurement in paediatric patients three years of age and older.
Plexiform Neurofibromas Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: | US$ 1,371.14 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 8.2% | 2030 Value Projection: | US$ 2,576.19 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
AstraZeneca, Pfizer Inc., Sun Pharmaceuticals Industries Limited, Mallinckrodt Pharmaceuticals, SpringWorks Therapeutics, Alcaliber S.A, Teva Pharmaceutical Industries Ltd., Glenmark, Amneal Pharmaceuticals LLC, Aurobindo Pharma, Apotex Inc., Mylan N.V., GSK plc., Solara Active Pharma Sciences Ltd., Abbott, Shanghai Kechow Pharma, Inc., Endo Pharmaceuticals Inc., Purdue Pharma L.P, Merck & Co., Inc., NFlection Therapeutics, Healx, and Array Biopharma |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Figure 2. Global Plexiform Neurofibromas Treatment Market Share (US$ Million), by Region, 2022
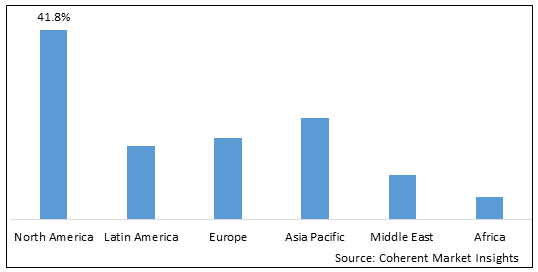
To learn more about this report, Download Free Sample
Global Plexiform Neurofibromas Treatment Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 can affect the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, U.A.E., Egypt, and others are facing problems with the transportation of drugs from one place to another.
The COVID-19 pandemic also had a negative impact on the global plexiform neurofibromas treatment market growth during COVID-19. Key stakeholders in the healthcare industry were impacted by the COVID-19 pandemic, which significantly disrupted clinical trial operations across the globe. Due to staff leaves, social-distance protocols, financial losses, and patient safety concerns, the investigative site's capabilities were disrupted. Companies that support the development of pharmaceuticals, including sponsors and contract research organizations, have shifted to remote working arrangements. Participation in clinical trials was affected due to COVID-19. According to half of clinics, existing clinical trial protocols were modified or postponed, and new research protocol delays or suspensions were reported. Even short-term delays in clinical trial research that devastated many neurofibromatosis families and slow Neurofibromatosis research aimed at developing new treatments, because the majority of Neurofibromatosis manifestations do not have approved treatment options.
Global Plexiform Neurofibromas Treatment Market: Key Developments
Increasing adoption of inorganic strategies such as product approval by regulatory authorities such as European Commission is expected to drive the gloal plexiform neurofibromas treatment over the forecast period. For instance, in June 2021, AstraZeneca Plc., a pharmaceutical company, announced that the Koselugo (selumetinib) had been granted conditional approval in the European Union for the treatment of symptomatic, inoperable plexiform neurofibromas in pediatric patients with neurofibromatosis type 1 aged 3 years and above. The approval by the European Commission was based on positive results from the SPRINT Stratum 1 Phase II trial sponsored by the National Institute of Health's National Cancer Institute Cancer Therapy Evaluation Program. The SPRINT Phase II trial indicated that Koselugo (selumetinib) reduced the tumor volume in children, reducing the pain.
Global Plexiform Neurofibromas Treatment Market: Restraint
Lack of approved drugs for the treatment of neurofibromatosis Type 1 is anticipated to hamper the growth of the global plexiform neurofibromas treatment market over the forecast period. For instance, in July 2022, according to the data published by the National Center for the Biotechnology Information, selumetinib developed by the AstraZeneca, a biopharmaceutical company, is the only drug approved by the U.S. Food and Drug Administration and European Commission for the treatment of pediatric patients aged 2 years and older suffering from neurofibromatosis type 1 who have inoperable, symptomatic plexiform neurofibromas (PN). Moreover, other symptomatic treatment are followed for the management of the disease.
Global Plexiform Neurofibromas Treatment Market - Key Players
Major players operating in the global plexiform neurofibromas treatment market include AstraZeneca, Pfizer Inc., Sun Pharmaceuticals Industries Limited, Mallinckrodt Pharmaceuticals, SpringWorks Therapeutics, Alcaliber S.A, Teva Pharmaceutical Industries Ltd., Glenmark, Amneal Pharmaceuticals LLC, Aurobindo Pharma, Apotex Inc., Mylan N.V., GSK plc., Solara Active Pharma Sciences Ltd., Abbott, Shanghai Kechow Pharma, Inc., Endo Pharmaceuticals Inc., Purdue Pharma L.P, Merck & Co., Inc., NFlection Therapeutics, Healx, and Array Biopharma
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients