Phenylketonuria Treatment Market Size and Forecast – 2025 – 2032
The Global Phenylketonuria Treatment Market size is estimated to be valued at USD 1.2 billion in 2025 and is expected to reach USD 2.1 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 8.5% from 2025 to 2032.
Global Phenylketonuria Treatment Market Overview
Phenylketonuria (PKU) treatment products are designed to manage the body’s inability to metabolize phenylalanine, an amino acid found in protein-rich foods. Core products include medical foods and phenylalanine-free formulas that provide essential nutrients while maintaining safe amino acid levels. Pharmaceutical options such as enzyme substitution therapies and synthetic tetrahydrobiopterin (BH4) formulations help reduce phenylalanine accumulation. Recent advancements include gene therapy research and extended-release formulations aimed at improving adherence and patient outcomes.
Key Takeaways
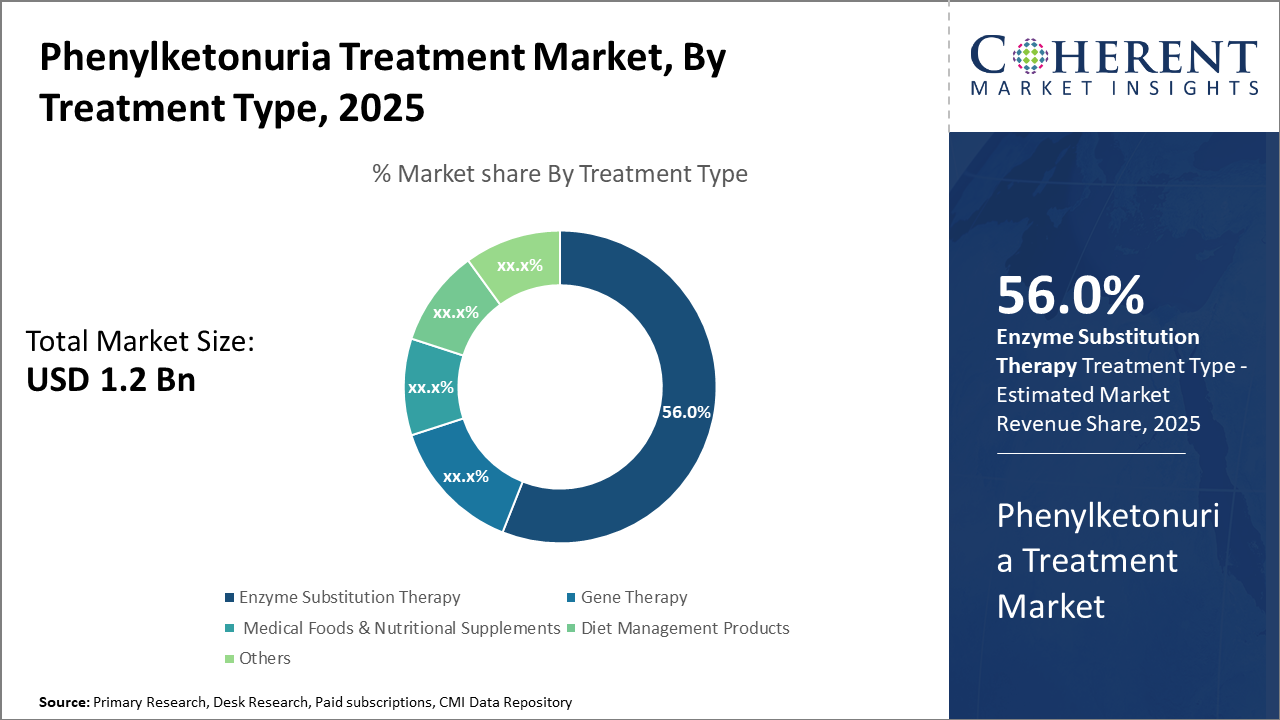
The enzyme substitution therapy segment dominates the Phenylketonuria Treatment Market, reflecting robust clinical validation and broad market adoption, cementing its 56% market share in 2024.
Pediatric patients represent the fastest-growing age group segment, propelled by increasing newborn screening and early treatment interventions.
Hospital pharmacies remain the primary distribution avenue, accounting for nearly half of the market share, supported by integrated patient care settings in developed regions.
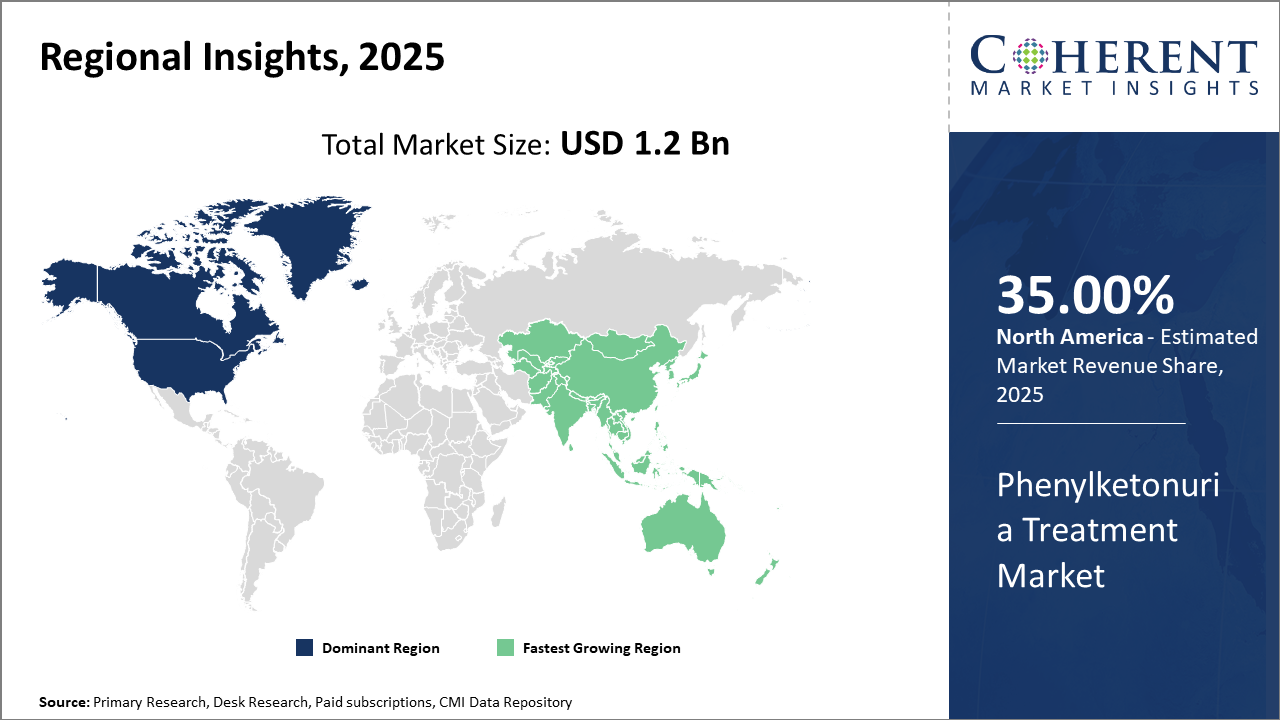
Regionally, North America holds the largest market share with 35% due to advanced healthcare infrastructure and government-led screening initiatives, while Asia Pacific exhibits the highest CAGR, supported by expanding diagnostics infrastructure and rising patient awareness.
Phenylketonuria Treatment Market Segmentation Analysis
To learn more about this report, Download Free Sample
Phenylketonuria Treatment Market Insights, By Treatment Type
Enzyme Substitution Therapy dominates the market share with 56% in 2024 due to its established efficacy and broad acceptance across age groups. This segment benefits from approved therapeutics like pegvaliase that have demonstrated real-world phenylalanine reduction success. Gene Therapy is the fastest growing subsegment, experiencing rapid advancements and clinical pipeline expansion. Its potential for long-term phenylalanine metabolism correction aligns with evolving therapeutic preferences toward durable treatments. Medical Foods and Nutritional Supplements maintain steady growth, supporting essential dietary management.
Phenylketonuria Treatment Market Insights, By Patient Age Group
The Pediatric segment dominates, supported by extensive newborn screening and early intervention strategies, representing the largest industry share in diagnosed cases. Infants and newborns follow with rapidly growing treatment initiation rates driven by universal screening programs. Adolescents are the fastest growing group, reflecting increasing therapeutic adherence facilitation and expanded treatment options beyond infancy.
Phenylketonuria Treatment Market Insights, By Distribution Channel
Hospital Pharmacies dominate with 48% market share, favored due to integrated healthcare approaches and multidisciplinary metabolic care units. Their infrastructure supports complex treatments like enzyme substitution administration and patient monitoring. Online Pharmacies emerge as the fastest growing channel, driven by digital health adoption and patient convenience trends, particularly post-pandemic. Retail Pharmacies and Specialty Clinics ensure localized access to medical foods and nutritional supplements, enhancing reach.
Phenylketonuria Treatment Market Trends
The market is witnessing notable trends such as the rise of gene editing therapies, which are advancing beyond conventional care to offer potentially curative solutions.
For example, ongoing trials in 2025 for gene editing therapies targeting PKU demonstrated a substantial, sustained reduction in phenylalanine levels.
Additionally, the integration of telemedicine and remote phenylalanine monitoring has gained traction, enhancing continuous patient care and adherence.
Pharmaceutical companies are increasingly investing in combination therapies that couple enzyme substitution with nutritional management, optimizing disease control.
Regionally, North America dominates this market, capturing approximately 40% of the market share by 2024, driven by well-established newborn screening mandates and reimbursement frameworks.
The USA's strong biotechnology sector, led by companies such as BioMarin and Synlogic, fuels market revenue growth as these firms rapidly advance therapeutic innovations.
Conversely, Asia Pacific stands out as the fastest-growing region, boasting a CAGR exceeding 9%, backed by expanding healthcare infrastructure in countries like China and India and increasing awareness programs that facilitate early diagnosis and treatment adoption.
Phenylketonuria Treatment Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Phenylketonuria Treatment Market Analysis and Trends
In North America, the Phenylketonuria Treatment Market dominance is largely attributed to comprehensive newborn screening programs covering over 98% of newborns, enabling early therapeutic intervention. Robust funding for rare diseases, facilitated through agencies such as the NIH, and strong presence of key market players amplify the market expansion. The established healthcare ecosystem encourages adoption of innovative enzyme substitution and gene therapies, leading to a significant market share.
Asia Pacific Phenylketonuria Treatment Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest market growth with a CAGR above 9%, underpinned by rapidly improving metabolic disease diagnosis frameworks in countries like China and India. Government initiatives to incorporate PKU screening in national newborn screening panels, coupled with rising investments in healthcare infrastructure, underpin this growth. Notably, the expansion of distribution channels by major market players in these regions is catalyzing accessibility and traction.
Phenylketonuria Treatment Market Outlook for Key Countries
USA Phenylketonuria Treatment Market Analysis and Trends
The USA’s Phenylketonuria Treatment Market is propelled by its advanced healthcare infrastructure and stringent newborn screening protocols mandated nationwide. Biopharmaceutical entities such as BioMarin Pharmaceutical dominate with FDA-approved enzyme substitution therapies showing widespread adoption. Analysis of 2024 healthcare data reveals a steady increase in enzyme therapy prescriptions, accounting for over 60% of the treatment use. The availability of patient assistance programs and insurance coverage further supports market growth, making the USA a key revenue contributor globally.
Germany Phenylketonuria Treatment Market Analysis and Trends
Germany's market demonstrates considerable potential driven by strong universal healthcare support and early metabolic abnormalities screening policies. The presence of specialty clinics focusing on inborn errors of metabolism contributes to rising market revenue, particularly in enzyme substitution and medical nutrition segments. In 2024, specialized medical nutrition products saw a 15% year-on-year growth, attributed to better patient compliance initiatives and reimbursement policies. Market players have optimized their local partnerships to enhance penetration in Germany, consolidating the region’s growth prospects.
Analyst Opinion
Advanced Diagnostic Penetration: The expansion of newborn screening programs, particularly in North America and Europe, acts as a crucial demand-side indicator accelerating market growth. For instance, by 2024, the USA screened over 98% of newborns for PKU, enabling early intervention and driving treatment adoption. This proactive identification has markedly increased the diagnosed patient pool, influencing the market size and revenue positively.
Expansion of Enzyme Substitution Therapies: Enzyme substitution therapies continue to represent the leading treatment segment in the PKU market, reflected by a 56% share in 2024. The availability of pegvaliase, an FDA-approved enzyme therapy, has expanded treatment options beyond dietary restrictions. Clinical data from a 2024 cohort study demonstrated a 40% improvement in phenylalanine tolerance in patients undergoing enzyme substitution, reinforcing this approach’s role in market growth.
Shifting Treatment Paradigms through Gene Therapy: Recent advancements in gene therapies, which aim at long-term correction of metabolic defects, are emerging as a key micro-indicator for future market potential. Ongoing clinical trials in 2025 reflect promising therapeutic efficacy, with early-phase results indicating a 30-35% reduction in phenylalanine levels sustained beyond six months post-treatment in trial subjects. These developments are expected to redefine market dynamics in the medium term.
Increasing Adoption of Medical Foods and Nutritional Supplements: Growth in medical nutrition tailored for PKU patients remains robust, driven by demand for safer, easy-to-consume formulations. For example, consumption of specialized amino acid formulas showed a 12% increase in Europe in 2024, backed by reimbursement policy enhancements. This demand-side momentum correlates with growing patient compliance and market revenue expansion.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 1.2 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.5% | 2032 Value Projection: | USD 2.1 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | BioMarin Pharmaceutical Inc., Synlogic, Inc., Nestlé Health Science, Kuvan® (BioMarin), PTC Therapeutics, Inc., LivaNova PLC, Cambrooke Therapeutics, Orphan Europe (Recordati Group), Synthon BV, Sobi (Swedish Orphan Biovitrum). | ||
| Growth Drivers: | Improved medical food formulations Enzyme replacement advancements |
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Phenylketonuria Treatment Market Growth Factors
The growth of the market is primarily propelled by increasing newborn screening rates worldwide, allowing for timely treatment and reducing disease complications. Emerging gene therapy techniques represent a paradigm shift in treatment, with promising clinical outcomes opening avenues for durable disease management. Rising awareness campaigns and enhanced diagnostic capabilities, particularly in emerging economies, are expanding the patient base and intensifying demand. Additionally, reimbursement policies and governmental incentives for rare diseases across North America and Europe strengthen market revenue potential. Development of palatable, convenient medical nutrition products additionally supports improved patient compliance, fostering sustained business growth within this specialized therapeutic segment.
Phenylketonuria Treatment Market Development
In July 2025, PTC Therapeutics announced the U.S. FDA approval of SEPHIENCE (sepiapterin), a novel oral therapy for the treatment of sepiapterin-responsive phenylketonuria (PKU). The approval includes a broad labeling covering both adult and pediatric patients aged one month and older, making it the first approved therapy to target this specific metabolic pathway in PKU management. SEPHIENCE works by restoring deficient tetrahydrobiopterin (BH4) levels, thereby enhancing phenylalanine metabolism. This milestone reinforces PTC Therapeutics’ leadership in rare disease innovation and expands treatment options for patients with limited existing therapies.
In March 2024, Eton Pharmaceuticals acquired the U.S. commercial rights to PKU GOLIKE from Relief Therapeutics, strengthening its portfolio in rare metabolic disorders. Key Players
Leading Companies of the Market
BioMarin Pharmaceutical Inc.
Synlogic, Inc.
Nestlé Health Science
Kuvan® (BioMarin)
PTC Therapeutics, Inc.
LivaNova PLC
Cambrooke Therapeutics
Orphan Europe (Recordati Group)
Synthon BV
Sobi (Swedish Orphan Biovitrum)
Competitive strategies among these companies include BioMarin’s expansion of its enzyme substitution product line coupled with extensive patient support programs, leading to over 20% growth in revenues in 2024. Meanwhile, Synlogic has accelerated its R&D pipeline focusing on gene therapy candidates, forming strategic alliances with biotechnology firms, facilitating rapid clinical development and market entry projections. Nestlé Health Science’s investment in personalized nutrition for PKU patients contributed to distribution channel expansion in Europe and the Asia Pacific, resulting in a 15% market share increase in medical foods in 2024.
Phenylketonuria Treatment Market Future Outlook
The future of the Phenylketonuria Treatment Indsutry lies in advanced biotechnological innovations and personalized medicine. Companies are expected to expand research into gene therapy, RNA-based therapeutics, and novel enzyme platforms that offer long-term correction of the underlying metabolic dysfunction. Partnerships between biotech firms, healthcare institutions, and patient advocacy organizations will drive faster regulatory approvals and improved accessibility. The integration of digital health tools for metabolic monitoring will enhance adherence and enable data-driven treatment optimization. Expanding healthcare coverage and rising global investment in rare disease research will create favorable conditions for the market’s sustained evolution.
Phenylketonuria Treatment Market Historical Analysis
The Phenylketonuria Treatment Market originated from nutritional therapy solutions, primarily low-phenylalanine medical foods developed to manage this rare metabolic disorder. Initially, treatment accessibility was limited due to low awareness and high costs, but government newborn screening programs and improved healthcare infrastructure broadened diagnosis and early intervention. Pharmaceutical innovation introduced enzyme replacement and cofactor therapies that provided alternatives to rigid dietary control. Over time, awareness campaigns, patient advocacy groups, and orphan drug legislations contributed to a more structured and regulated market environment. The entry of biopharmaceutical companies specializing in rare diseases further diversified treatment options and improved patient outcomes.
Sources
Primary Research interviews:
Geneticists
Pediatric Neurologists
Metabolic Disorder Specialists
Pharmaceutical Researchers
Databases:
NCBI Genetic Disorders Database
WHO Rare Disease Database
Magazines:
Genetic Engineering & Biotechnology News
Rare Disease Report
Pharma Times
BioWorld
Journals:
Molecular Genetics and Metabolism
Orphanet Journal of Rare Diseases
Journal of Inherited Metabolic Disease
Newspapers:
The Guardian (Health)
The Economic Times (Biotech)
The New York Times (Science)
Hindustan Times (Health)
Associations:
National PKU Alliance
Genetic Alliance
European Society of Human Genetics
National Organization for Rare Disorders
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients