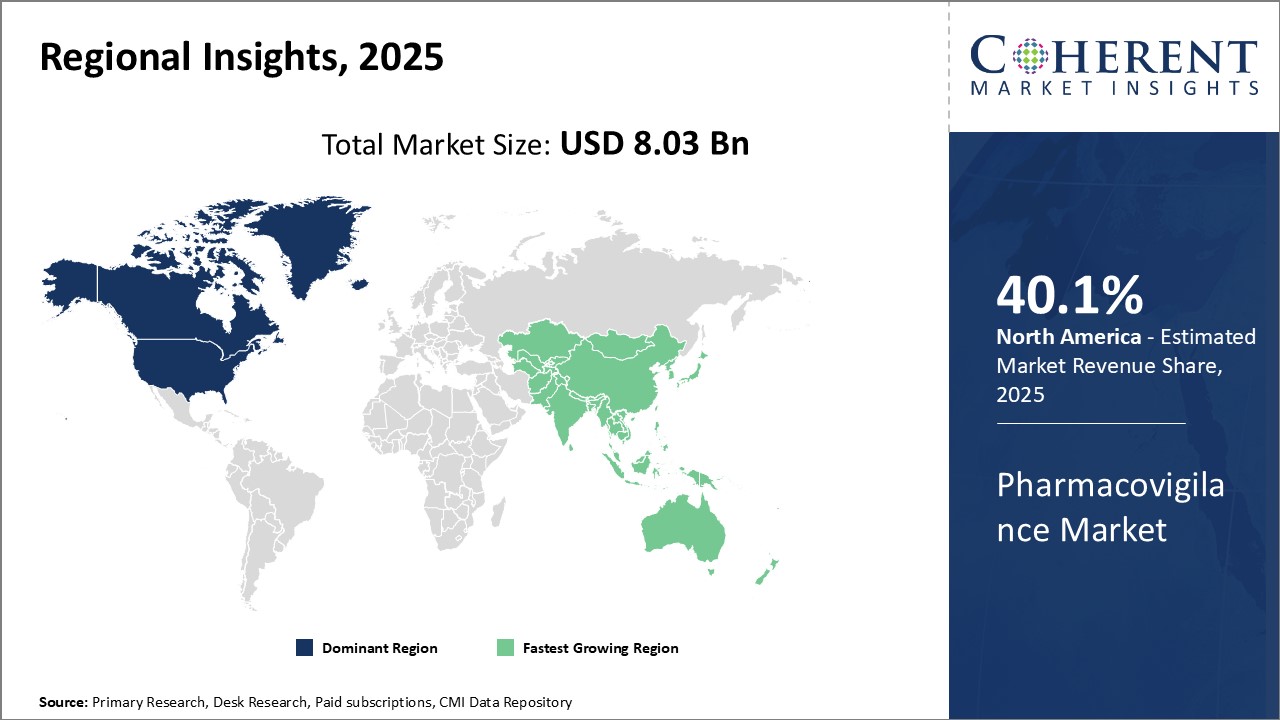
The global pharmacovigilance market is estimated to be valued at USD 8.03 Bn in 2025 and is expected to reach USD 14.03 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 8.3% from 2025 to 2032.
To learn more about this report, Download Free Sample
Pharmacovigilance Market demand is mainly fueled by the growing requirement for monitoring drug safety, particularly in the aftermath of heightened global consumption of drugs and regulatory oversight. As drug development and approval procedures become increasingly complicated, the necessity for efficient pharmacovigilance practices becomes more urgent than ever.
Pharmacovigilance services, encompassing adverse event reporting, risk management, and signal detection, are becoming essential parts of the healthcare environment.
|
Current Events |
Description and its impact |
|
Increased Regulatory Scrutiny |
|
|
Integration of AI and Automation |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Artificial Intelligence (AI) plays a transformative role in the pharmacovigilance industry by automating and enhancing key drug safety processes. It accelerates adverse event detection, literature monitoring, and case processing by quickly analyzing vast datasets with high accuracy. AI-powered tools reduce manual workload, minimize human error, and enable faster decision-making, improving overall compliance and patient safety. Natural language processing (NLP) helps extract relevant information from unstructured sources, while machine learning models identify patterns and predict potential risks.
In July 2025, EVERSANA launched EVERSANA ORCHESTRATE PV, an AI-driven solution designed to streamline pharmacovigilance operations. The platform enhances key workflows like literature monitoring and aggregate reporting, delivering improved speed, accuracy, and quality. It automates and customizes processes for scalability, cutting operational timelines by up to 50% and reducing manual effort by 40%.
To learn more about this report, Download Free Sample
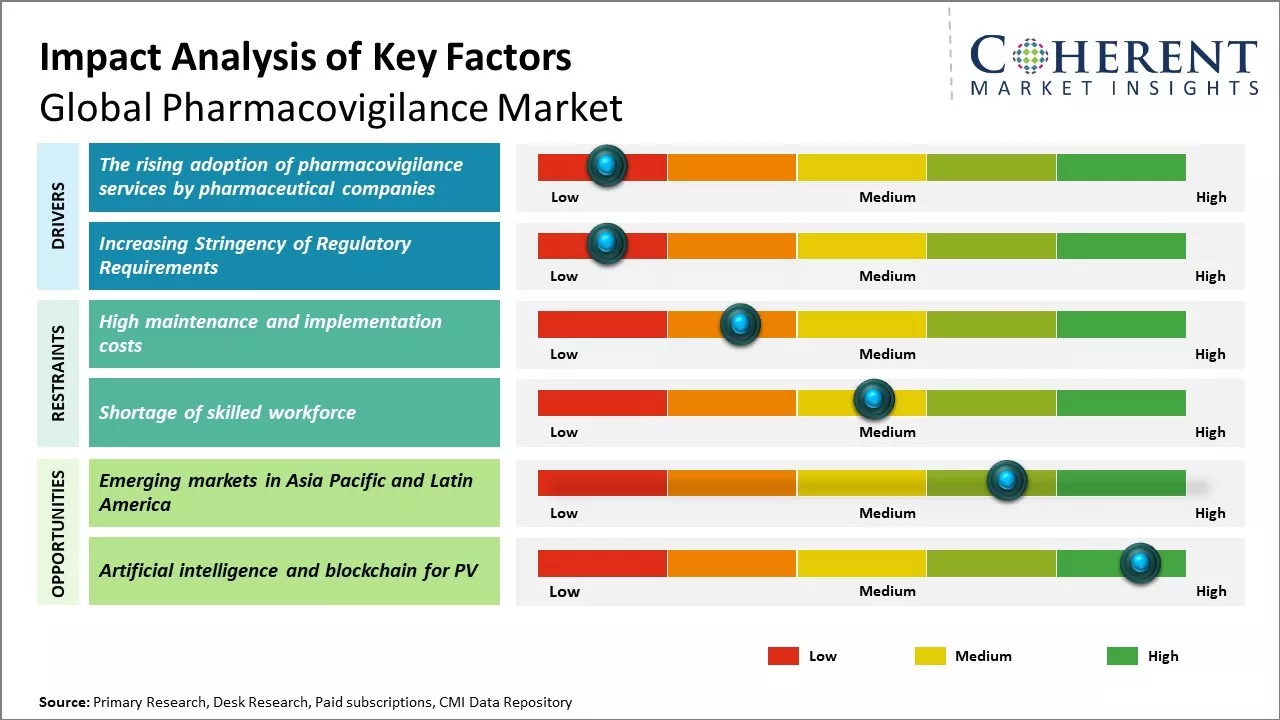
Pharmacovigilance services adoption by pharmaceutical organizations is increasing heavily because of stringent regulations for ensuring drug safety. Regulatory authorities such as FDA and EMA are imposing strict norms to monitor and ensure drug safety after marketing. This is pushing pharmaceutical organizations to outsource pharmacovigilance activities to expert Clinical Research Organizations (CROs) who possess experience and infrastructure to perform the services efficiently.
Pharmacovigilance outsourcing helps drug companies keep their core capabilities of drug discovery and development at the forefront, while leaving monitoring activities for drug safety to experts in CRO partners. Pharmaceutical companies gain advantage through access to cutting-edge technology, trained people and a combined global strategy toward safety reporting.
In 2024, ProPharma Group purchased Clinres Farmacija, a European-based clinical trial and pharmacovigilance firm. This purchase enhances ProPharma's pharmacovigilance service capabilities, adding to its post-market regulatory strengths.
The increasing stringency of regulatory requirements across various countries is expected to drive significant growth in the global pharmacovigilance market. With new drugs entering the market at an unprecedented rate and increasing complexity of drug safety profiles, regulatory authorities have instituted more rigorous legislation and safety standards.
Pharmaceutical companies are now mandated to conduct comprehensive safety profiling of drugs right from the early stages of development along with continual monitoring even post marketing. Compliance with regulatory norms has become absolutely critical for continued drug approvals and market access. The healthcare industry has also undergone intense scrutiny in the aftermath of several high-profile drug safety issues and failures.
The emerging markets in Asia Pacific and Latin America present a huge growth opportunity for the global pharmacovigilance market. These regions are experiencing rapid economic development which has lifted billions of people out of poverty and expanded the middle-class consumer base with increased spending power. As disposable incomes rise, healthcare systems are also undergoing modernization with greater investment.
This has resulted in larger patient populations gaining access to advanced medications for various chronic and life-threatening diseases. At the same time, the growth of the pharmaceutical industry has exploded in these regions to cater to the demands of their huge populations. Many large multinational drug manufacturers have set up manufacturing and R&D facilities within Asia Pacific and Latin America in recent years to reduce costs and be closer to their customer base.
The clinical trial phase III segment is expected to contribute highest share in 2025 owing to stringent regulatory requirements. Phase III trials involve several hundred to thousands of human participants and are intended to confirm the overall risk-benefit relationship of a drug and provide an adequate basis for labeling.
Regulatory authorities such as the USFDA and EMA have implemented extensive regulations for phase III trials to ensure drug safety before market approval. Sponsors are required to develop comprehensive risk management plans to monitor adverse events throughout the trial period. This involves maintaining robust pharmacovigilance systems to detect, assess, and prevent drug safety issues from harming participants.
By Type of Method, Spontaneous Reporting Segment holds the largest market share. This is due to its wide acceptance. Traditionally, spontaneous reporting involved collecting adverse event reports using paper-based forms submitted voluntarily by healthcare professionals and consumers.
However, regulatory agencies are now pushing for electronic reporting standards to modernize pharmacovigilance practices. Mandates like E2B and ICH E2B(R3) specify structured format requirements and technical specifications for electronic transmission of individual case safety reports (ICSRs). Compliance with these standards enables centralized interoperable databases and advanced data analytics capabilities at the global level.
The in-house segment has the largest share of the pharmacovigilance market in 2025 owing to tangible cost-control advantages over outsourcing. In-house teams manage drug safety operations using a sponsor's own resources and facilities without involving third parties. Retaining pharmacovigilance capabilities internally offers direct oversight of processes to ensure strategic business priorities are addressed.
To learn more about this report, Download Free Sample
North America has established itself as the dominant region in the global pharmacovigilance market. The region is home to some of the largest pharmaceutical companies in the world as well as many contract research organizations (CROs) specializing in pharmacovigilance services. Stringent regulatory requirements for drug safety monitoring imposed by regulatory bodies such as the FDA have driven significant investments by pharmaceutical and biotech firms in building robust pharmacovigilance systems.
The Asia Pacific region has emerged as the fastest growing market for pharmacovigilance industry globally. This can be attributed to rising generics production, expanding clinical trials by multinational players and growing expertise of local CROs. India, in specific, has emerged as a world sourcing destination for pharmacovigilance services because of the availability of low-cost but skilled manpower.
Some of the major pharmaceutical corporations have set up regional pharmacovigilance centers or collaborated with Indian CROs in order to concentrate post-marketing safety activities. At the same time, drug regulators in Asia Pacific have also been enhancing regulations in connection with pharmacovigilance and encouraging safety best practices.
United States dominates the pharmacovigilance market at the global level, adding a large contribution in terms of share owing to its well-established pharma sector and stringent regulation system. United States Food and Drug Administration (FDA) strictly ensures comprehensive practices in pharmacovigilance through its strict policies, boosting demand for safety monitoring services.
Presence of multinational pharmaceutical firms as well as Contract Research Organizations (CROs) further contributes to the boost, and therefore, the U.S. takes a leadership role in pharmacovigilance activity. Also, the increased uptake of new technologies like artificial intelligence and big data analytics is adding fuel to the market growth.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 8.03 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.3% | 2032 Value Projection: | USD 14.03 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
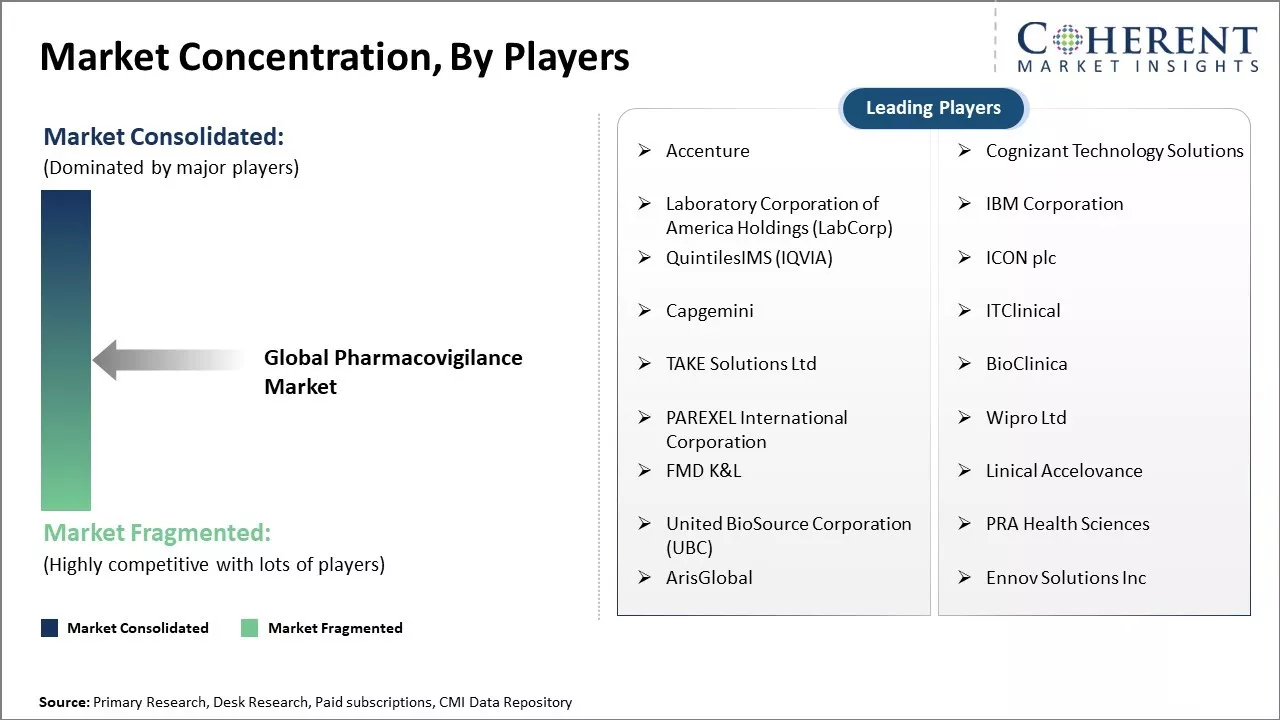
Accenture, Cognizant Technology Solutions, Laboratory Corporation of America Holdings (LabCorp), IBM Corporation, QuintilesIMS (IQVIA), ICON plc, Capgemini, ITClinical, TAKE Solutions Ltd, BioClinica, PAREXEL International Corporation, Wipro Ltd, FMD K&L, Linical Accelovance, United BioSource Corporation (UBC), PRA Health Sciences, ArisGlobal, and Ennov Solutions Inc |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: The global pharmacovigilance market refers to the process of detection, assessment, understanding, and prevention of adverse effects associated with drugs and medical products. It involves monitoring the effects of medical drugs after they have been approved for marketing, especially looking into adverse effects and documenting information about hazards. The global pharmacovigilance market generally consists of drug manufacturers, contract service providers, regulatory authorities like FDA that monitor the safety of marketed drugs and medical devices. Timely identification and management of risks related to drug safety helps minimize health issues and protects patients.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients