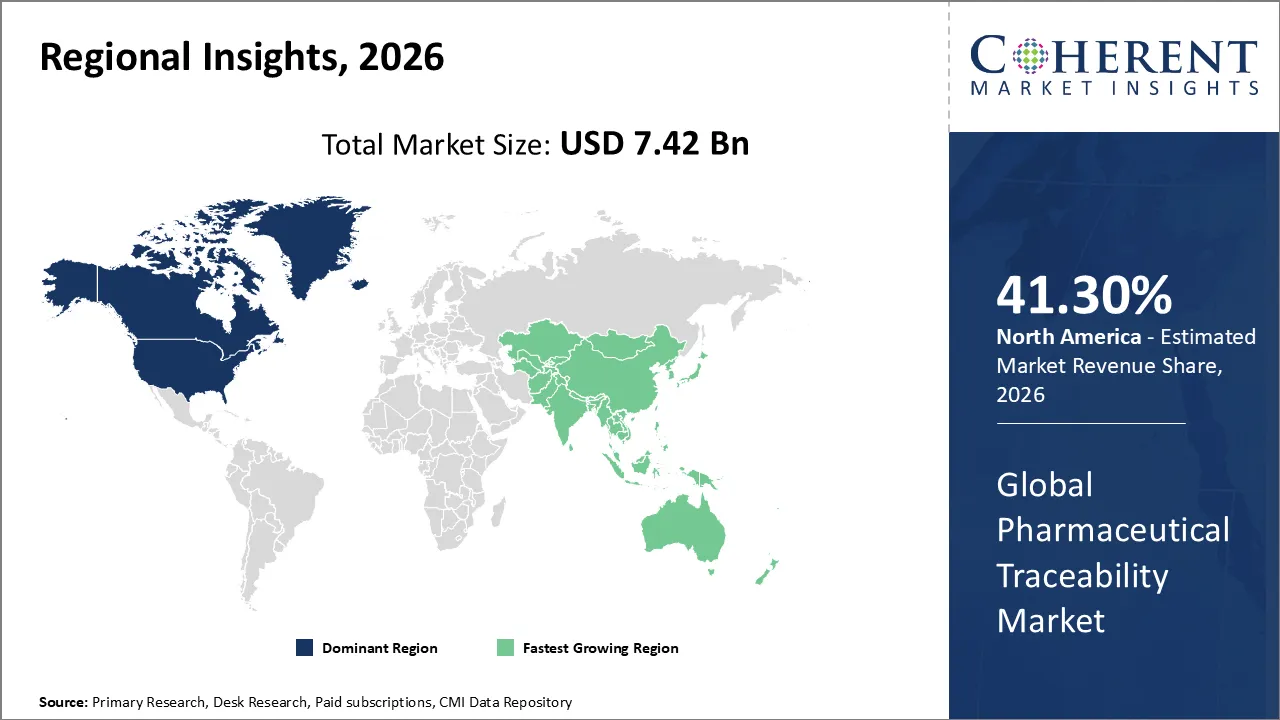
The Pharmaceutical Traceability Market is estimated to be valued at USD 7.42 Bn in 2026 and is expected to reach USD 22.5 Bn by 2033, exhibiting a compound annual growth rate (CAGR) of 18.4% from 2026 to 2033.
The pharmaceutical traceability market enables companies to actively track and verify medicines throughout the supply chain, from manufacturing to dispensing. Industry players adopt these systems to enhance patient safety, combat counterfeit drugs, and meet regulatory requirements. Technologies such as serialization, barcoding, RFID, and integrated software platforms provide real-time visibility, streamline recalls, and improve inventory control. Increasing supply chain complexity, ongoing digital transformation, and stricter regulatory oversight continue to drive global market growth.
|
Current Events |
Description and its impact |
|
Regulatory Advances in Pharmaceutical Serialization |
|
|
Technological Innovations in Traceability Solutions |
|
|
Economic Pressures and Healthcare Reforms |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Positioning System (GPS) hold the largest market share of 36.6% in 2026. The pharmaceutical traceability market increasingly relies on Global Positioning System (GPS) technology to enable real-time shipment monitoring and stronger logistics control. Stakeholders use GPS to actively track the precise location of pharmaceutical products during transit, minimizing risks such as theft, diversion, and delays. GPS also improves coordination across complex distribution networks, reinforces cold-chain integrity, and enables faster responses to disruptions, enhancing overall supply chain reliability, transparency, and efficiency.
The Tripura government unveiled 21 e-hospitals and deploy 50 life-support ambulances equipped with GPS tracking systems as part of its efforts to modernize healthcare services across the state.
Hospitals acquired the prominent market share of 34.6% in 2026. Hospitals actively fuel the pharmaceutical traceability market by focusing on safe medication practices and streamlined clinical operations. They use traceability systems to track drug movement within their facilities, confirm product authenticity before administration, and minimize medication errors. These systems also enable precise inventory monitoring, better expiration control, and quick identification of recalled products. As hospitals expand the use of digital health records and automated pharmacy solutions, traceability tools enhance workflow efficiency, accountability, and patient care quality. For instance, in August 2025, LSPedia has partnered with Spring Bio Solution to strengthen pharmaceutical traceability in India. Through this collaboration, Spring Bio Solution will support LSPedia’s global expansion and accelerate adoption of its compliance, serialization, and supply chain software solutions.
To learn more about this report, Download Free Sample
North America dominates the overall market with an estimated share of 41.3% in 2026. Strong regulatory frameworks actively drive North America’s pharmaceutical traceability market, prompting companies to adopt advanced tracking technologies across the supply chain. Businesses increasingly integrate digital solutions such as IoT, blockchain, and cloud-based platforms to improve visibility and enable seamless data sharing. Healthcare providers and manufacturers focus on anti-counterfeiting measures and real-time monitoring to safeguard patient safety. By collaborating across the industry and investing in automation, stakeholders actively enhance traceability practices and boost operational efficiency throughout the region.
In June 2025, Loma Systems has launched the X5DE Dual Energy X-ray Inspection System in North America, offering manufacturers enhanced confidence and control through features such as reject confirmation, automatic image archiving, and full digital traceability.
Regulators and industry players in the Asia Pacific actively advance the pharmaceutical traceability market by emphasizing supply chain transparency and drug safety. Countries including China, India, Japan, and Southeast Asian nations enforce stronger serialization and tracking requirements to meet global standards and prevent counterfeit medicines, driving adoption of digital technologies such as RFID, cloud platforms, and blockchain. Rapid pharmaceutical manufacturing, expanding healthcare infrastructure, and growing e‑commerce actively promote the deployment of traceability solutions throughout the region. For instance, in October 2025, the Central Drugs Standard Control Organisation launched a digital tracking system to monitor the supply chain and quality of high-risk pharmaceutical solvents, including diethylene glycol.
U.S. stakeholders actively shape the pharmaceutical traceability market by implementing the Drug Supply Chain Security Act (DSCSA), driving widespread serialization and end-to-end tracking across manufacturers, distributors, and pharmacies. Companies deploy digital solutions such as cloud-based platforms, RFID, and real-time tracking to improve supply chain transparency and prevent counterfeit drugs, while healthcare providers integrate traceability with patient safety systems. Ongoing innovation and regulatory oversight actively promote the adoption of advanced tracking practices across the nation. For instance, in December 2025, SMX PLC has expanded its industrial rubber traceability platform to include latex and rubber gloves used across healthcare, laboratory, pharmaceutical, food-handling, industrial, and consumer sectors.
Manufacturers and regulators in India are actively advancing the pharmaceutical traceability market by emphasizing drug safety, authenticity, and supply chain transparency. The government mandates QR codes on key drug formulations and expands serialization to APIs and other products, promoting digital tracking across the supply chain. Companies implement smart packaging, barcoding, and integrated systems to comply with domestic and international standards, prevent counterfeit medicines, and strengthen global market access for Indian pharmaceuticals.
The pharmaceutical traceability market is increasingly driven by digital technologies, including IoT sensors, cloud platforms, and mobile applications. Companies deploy these solutions to monitor shipments, environmental conditions, and inventory in real time. IoT integration allows automated data collection, reduces human error, and supports predictive analytics, enabling stakeholders to make faster, data-driven decisions. This trend enhances transparency, strengthens cold-chain management, and streamlines operations, positioning digital-enabled traceability as a critical enabler of modern pharmaceutical supply chains.
Blockchain technology is emerging as a major trend, offering secure, immutable records of pharmaceutical product movement. By recording transactions at each supply chain stage, blockchain reduces the risk of counterfeiting and diversion while providing stakeholders with verifiable product history. Pharmaceutical companies increasingly implement blockchain to meet regulatory demands, ensure patient safety, and improve trust across distributors, hospitals, and pharmacies. This technology supports automated verification and audit processes, making traceability more transparent and reliable.
The market offers opportunities for integrating traceability with advanced technologies such as IoT, AI, blockchain, and cloud computing. These integrations provide real-time monitoring, predictive analytics, and secure data sharing, improving efficiency and decision-making across the supply chain. Companies that develop platforms combining multiple digital technologies can offer end-to-end traceability, reduce operational risks, enhance compliance reporting, and provide superior value to manufacturers, distributors, and healthcare providers, driving adoption across global pharmaceutical networks.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 7.42 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 18.4% | 2033 Value Projection: | USD 22.5 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Optel Ltd., The Healthcare Distribution Alliance (HDA) Inc., Bureau Veritas, Adents, Logista Pharma, rfxcel Corporation, Movilitas Consulting AG, Trace Link Inc., Avery Dennison Corporation, Pharmalutions Pte Ltd., and Cognex Corporation. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients