The Pharmaceutical Testing Services Market is estimated to be valued at USD 20 Bn in 2026 and is expected to reach USD 37.0 Bn by 2033, growing at a compound annual growth rate (CAGR) of 9.2% from 2026 to 2033.
This sector encompasses testing services provided to pharmaceutical and biopharmaceutical companies throughout drug development and manufacturing. The growing complexity of drug molecules and increasing regulatory scrutiny are driving the market value for pharmaceutical testing services.
The Pharmaceutical Testing Services Market is trending towards the greater outsourcing and specialization of testing capabilities. Pharmaceutical companies are embracing contract testing laboratories and CROs that provide comprehensive analytical services integrated with regulatory expertise for enhanced compliance. Specialized testing for biologics and biosimilars is also growing in popularity over traditional small molecule testing. This is because they provide improved quality assurance, advanced characterization, and deeper insights into drug safety and efficiency profiles. This outsourcing shift will continue propelling market volumes higher over the forecast period.
|
Current Event |
Description and the Impact |
|
Technological Advancements in Testing Methodologies |
|
|
Regional Public Health Crises and Emerging Disease Outbreaks
|
|
|
Environmental and Sustainability Regulations Impacting Laboratory Operations
|
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Artificial intelligence (AI) is reshaping the pharmaceutical testing services market in a major way. drug discovery and development are being done at a much faster pace and with greater efficiency due to AI algorithms. These algorithms analyze a large amount of data to find potential drug candidates, forecast their effectiveness and toxicity, and even figure out the best clinical trial designs, thus cutting both time and money to market. AI is also at the forefront in handling and making sense complicate biological data, which in turn helps in the personalized medicine approaches. AI-driven automation in lab operations, eliminates human error and also greatly enhances the speed of the testing processes. AI is the main factor behind the market's expansion, as it is giving rise to more accurate, efficient, and cost saving testing services.
For instance, the McKinsey Global Institute (MGI) anticipated that AI could add USD 60 to USD 110 billion to the economy each year for the pharmaceutical and medical product industries. This advantage is because AI can speed up the process of finding compounds that could be used to make new drugs, speed up their development and approval, and make them easier to market.
In terms of service type, the bio-analytical testing segment contributes the highest share of 29.3% in 2026 of the market. The segment is growing because of increasing investment in new drug research, particularly for biological medicines that require detailed analysis of how drugs behave in the body. The strict rules from agencies like the FDA require extensive testing before drugs can be approved, thus propelling demand for these services.
For instance, in December 2022, Alliance Pharma, a world leader in the pharmaceutical and biopharmaceutical business that offers bioanalytical, DMPK, and CMC testing services, launched its new 20,000-square-foot bioanalytical laboratory, Alliance Pharma Pty, Ltd., in Brisbane, Australia. The company revealed plans earlier this summer to build a new facility in Australia, which will have a big lab to offer much-needed small and large molecule bioanalytical services to a region with few options for bioanalytical support.
In terms of product type, the finished products segment contributes the highest share of 47% in 2026 of the market owing to stringent regulatory batch release requirements and quality compliance protocols. Before being sold, finished products must undergo extensive testing and certification. This process establishes quality assurance frameworks that prioritize ready-to-market formulations. Such regulatory oversight ensures that finished goods comply with GMP standards prior to their sale.
In terms of end user, the biopharmaceutical and pharmaceutical companies contribute the highest share of 63.6% in 2026 of the market owing to stringent regulatory requirement and complex drug development pipelines. These companies outsource testing to specialized laboratories to ensure product safety, efficacy and compliance with FDA and other regulatory standards.
For instance, in November 2025, Servier India, a wholly owned subsidiary of Servier Group, introduced a biomarker testing program for two specific types of cancer. This was developed in collaboration with the genomic laboratories MedGenome and Strand Life Sciences based in India.
To learn more about this report, Download Free Sample
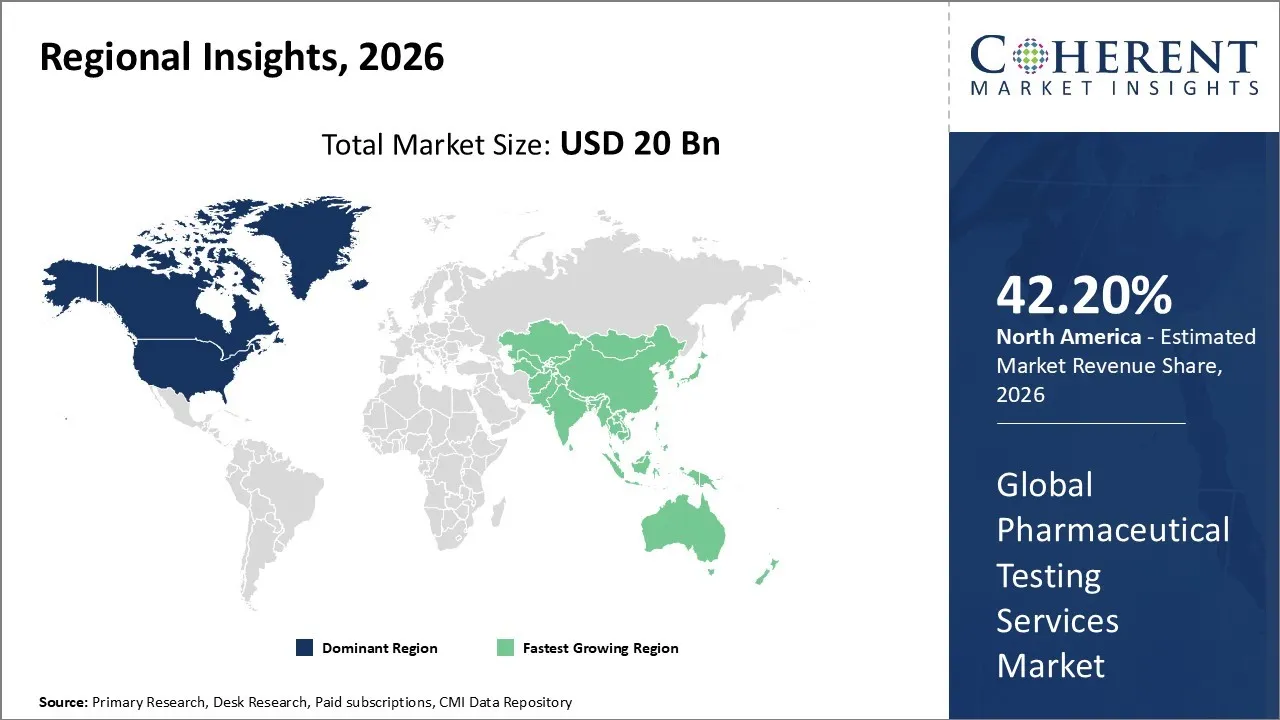
North America has remained the dominant region with 42.20% in 2026 of the global Pharmaceutical Testing Services Market over the past decade. The growth is attributable to the rising drug complexity, strict FDA regulations, increased outsourcing to CROs, and high R&D spending, particularly for new therapies and biosimilars. The region's strong infrastructure, along with its use of cutting-edge technology and a growing number of clinical trials, is also driving up the need for specialized analytical services. Two advanced analytical methods, mass spectrometry and chromatography, are essential for accurately describing biologics, biosimilars, and combination drugs.
For instance, in January 2024,Kindeva Drug Delivery, a provider of drug-device combination products, extended its analytical services capabilities by introducing a new global business unit. This unit provides integrated and independent analytical support to the pharmaceutical, biopharmaceutical, and medical device industry.
The Asia Pacific region has become the fastest growing market owing to increasing investment in research activities and a heightened demand for advanced testing of both biologics and generics. The pharmaceutical industry is growing quickly in cities like China, India, and Japan, which is driving up the need for testing new drugs for discovery and production. Greater investments in drug discovery, particularly in advanced therapies like biologics, require more sophisticated testing methods. The stricter quality and safety standards are fostering the need for extensive analytical assessments. Many global firms prefer to outsource testing to this region owing to its competitive costs and skilled workforces. Technologies like AI, automation, and digital platforms are improving the testing efficiency and accuracy. The rise in generics and biologics is resulting in high demand for specialized testing. The supportive government policies like "Make in India" are fostering domestic laboratory growth in the region.
For instance, in October 2024, Agilus Diagnostics unveiled its new Pharmacogenomics Testing Services. This service provides individuals and healthcare professionals with valuable information about how unique genetic profiles influence the effectiveness and safety of medications. This facilitates the development of a more personalized treatment plan.
The U.S. market growth is owing to strict FDA regulations, rising outsourcing to CROs, increased R&D for complex biologics, and demand for cutting edge technologies for quality and safety. Other factors like increased drug approvals, personalized medicine trends, greater complexity in drug development, and focus on data integrity, is also boosting the demand for specialized bioanalytical and stability testing services.
For instance, in May 2025, Minaris Regenerative Medicine was merged with the U.S. and U.K. operations of WuXi Advanced Therapies by the by New York based investment company, Altaris to form Minaris Advanced Therapies™. As a CDMO and testing expert in cell therapies globally the company headquartered in Pennsylvania provides the latest technology platforms, cell therapy and viral vector manufacturing, as well as comprehensive testing services.
China's market is growing owing to the rising R&D, increased clinical trials, and supportive government policies simplifying regulations. This makes China a global hub for drug development and attracting outsourcing from international firms, alongside a focus on novel therapies like gene editing.
For instance, in November 2023, Asahi Kasei and Asahi Kasei Medical will open the Asahi Kasei Bioprocess Technical Center (CBTC) in Suzhou, Jiangsu, China. The bioprocess division of Asahi Kasei Medical, which includes products for biotherapeutics, biosafety testing services, and a biopharmaceutical CDMO, is one of the Asahi Kasei Group's "10 Growth Gears" (GG10) businesses that will help the company grow in the future.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 20 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 9.2% | 2033 Value Projection: | USD 37.0 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Merck KGaA, Boston Analytical, West Pharmaceutical Services Inc., Exova Group PLC., Source BioScience , Pace Analytical Services, Inc., W.uXi AppTec, Toxikon, Eurofins Scientific, and West Pharmaceutical Services Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Today, the pharmaceutical industry is making substantial investments in the development of biologics and biosimilars. These complex molecules require extensive testing protocols during their entire lifecycle. Biologic drugs now account for a substantial share of new drug approvals, with manufacturers allocating heavily in research initiatives. Biologics, unlike traditional small-molecule drugs, require specialized analytical methods to guarantee consistency and safety throughout production batches.
For instance, in October 2025, the U.S. Food and Drug Administration (FDA) said it would take big steps to speed up and lower the cost of making biosimilar medicines. These are lower-cost generic versions of biologic drugs that treat serious and chronic diseases. This brave move by the FDA speeds up the development of biosimilars, increases competition in the market, and gives patients more choices.
A growing number of pharmaceutical companies are working with specialized CROs that utilize cutting-edge technology. This trend is creating numerous growth prospects in the testing services sector. These specialized CROs are skilled in areas like biomarker analysis, companion diagnostics, and complex biologics testing which are essential for modern drug development. The cutting-edge technology platforms like high throughput screening, mass spectrometry, and real world data analytics allow specialized CROs to provide faster and more accurate results. Their investment in cutting-edge infrastructure allows pharmaceutical firms to access sophisticated testing capabilities without major capital expenditure. Precision medicine and complex therapies, such as cell and gene therapies, are gaining popularity. These therapies require specialized testing that is not available at standard laboratories. This trend is particularly strong in oncology, rare diseases, and biosimilars development.
For instance, in October 2025, MilliporeSigma, Merck KGaA's life science business in the U.S. and Canada, teamed up with Promega Corporation, a global leader in life science solutions and services from Madison, Wisconsin, to work together on new technologies that will help with drug screening and discovery.
*Definition: The Pharmaceutical Testing Services Market facilitates quality assurance and regulatory compliance throughout the drug development lifecycle. It provides analytical testing solutions that allow pharmaceutical and biopharmaceutical companies to evaluate drug safety, efficacy, and quality in a reliable, accurate, and efficient way. Companies can use this market to conduct stability testing, validate manufacturing services, perform bioanalytical studies, ensure regulatory compliance, and verify product quality across global manufacturing sites. Testing services include analytical chemistry, microbiology testing, method development and validation, and emerging specialized services for complex biologics.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients