Global pharmaceutical manufacturing software market is estimated to be valued at USD 3.38 Bn in 2025 and is expected to reach USD 6.18 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 9.0% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
The market growth is driven by rising need for software that can manage increased regulations and complexity in pharmaceutical manufacturing and supply chain processes. Global pharmaceutical manufacturing software market trend includes increasing demand for cloud-based solutions and analytics functionalities. Due to rising need for real-time processing, remote monitoring, and data-based decision making, pharmaceutical manufacturers are adopting software-as-a-service models and integrating analytics tools to gain insights across production facilities and workflows. This allows improved quality oversight, waste reduction, and regulatory compliance.
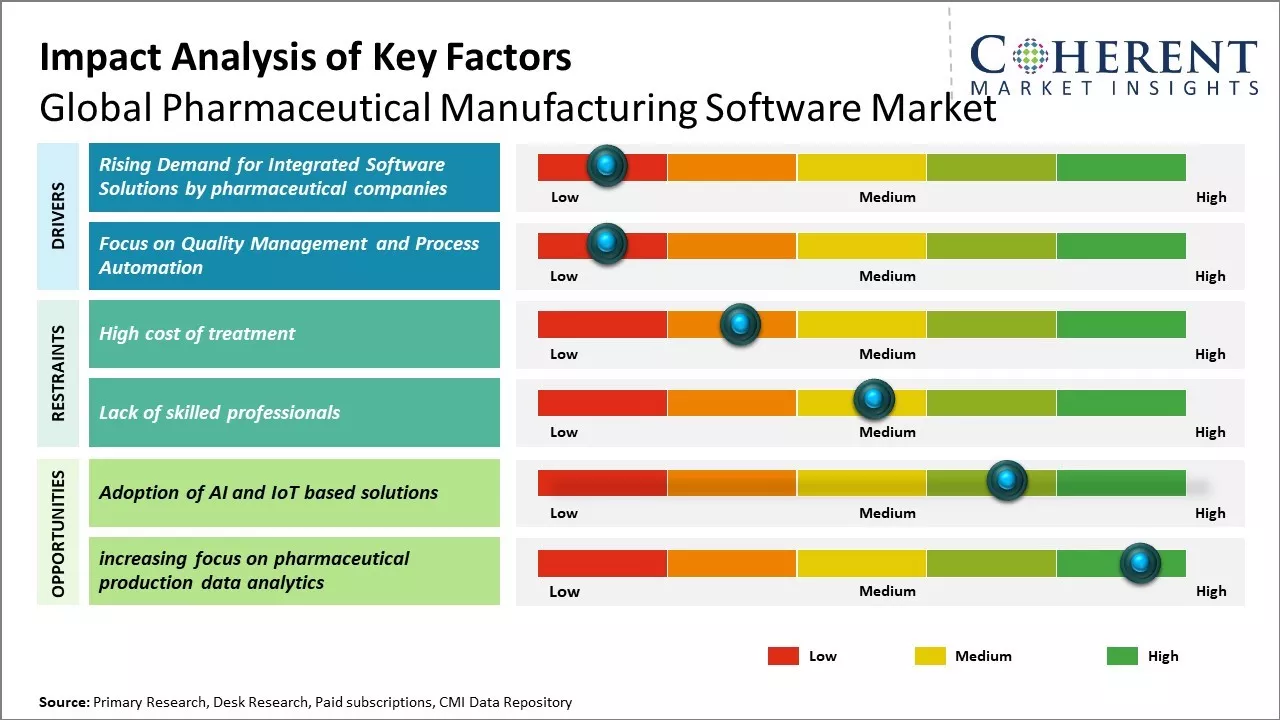
Market Driver: Rising Demand for Integrated Software Solutions by pharmaceutical companies
Growing pharmaceutical industry can boost demand for integrated software solutions among manufacturers that can help streamline operations and drive efficiencies. Traditional point solutions are no longer sufficient, as manufacturers seek holistic platforms that can support their needs across the supply chain from research and development to manufacturing and distribution. An integrated software suite allows visibility across functional silos and helps facilitate collaboration between departments. It also ensures compliance with ever-changing regulations through centralized data management and reporting features. This helps to reduce costs and optimize processes in a challenging business environment. Leading pharmaceutical software vendors have recognized this shifting priority and offers broader platforms through acquisitions or by building add-ons to their existing solutions. Integrated platforms that deliver increased oversight, agility and compliance can influence buyers in this industry. For instance, in January 2022, Aizon, a software company, introduced its brand-new asset tracking software for biotech and pharmaceutical firms. Based on Aizon's AI SaaS Platform that complies with GxP, Aizon Asset Health offers proactive real-time asset condition monitoring, intelligent historical maintenance analysis, potential problem detection, and actionable maintenance recommendations to maintain equipment in optimal operating condition.
Get actionable strategies to beat competition: Download Free Sample
Focus on Quality Management and Process Automation
Quality has always been paramount in pharmaceutical manufacturing due to the sensitive nature of medical products. However, regulatory requirements and customer expectations around quality are becoming more stringent. Thus, operational complexities are mounting with the rise of biologics and personalized medicines. This propel manufacturers to invest more aggressively in quality management software and process automation technologies. Applications that can digitize quality procedures, provide real-time production data and facilitate batch traceability are crucial for maintaining consistency and reducing compliance risks. Pharmaceutical firms also seek tools for automating repetitive tasks and incorporating quality checks to minimize human errors. Equipment monitoring and process analytical technologies support data-driven decision making. The pressing need to fortify quality systems while coping with a volatile operating climate boosts adoption of these solutions across the industry.
Key Takeaways from Analyst:
Global pharmaceutical manufacturing software market is expected to witness significant growth in the near future. Stringent regulations around maintaining quality standards in pharmaceutical production can boost demand for compliance software solutions that can help automate processes and enable digital documentation. Ensuring drug safety and efficacy throughout the product lifecycle from research to distribution requires capturing and analyzing vast amounts of data, thus, offering opportunities for analytics and process optimization tools.
North America currently dominates the market, owing to presence of many pharmaceutical giants and focus on cutting-edge technologies. However, growing generics markets in Asia and emerging regulations in Latin America can boost adoption in these regions. The economic slowdown due to the pandemic caused some near-term budget constraints for small and medium businesses. Furthermore, barriers around data integration can pose challenges for vendors in establishing comprehensive digital ecosystems.
Collaboration between pharma companies and software providers will be key to developing innovative solutions for complex industry needs such as personalized medicine and niche therapeutics production. Successful vendors will need to demonstrate deep domain expertise, flexibility to changing standards, and abilities to scale technologies across global operations. With manufacturing becoming increasingly knowledge-based, there will be significant demand for integrated platforms that connect plant floors with decision makers.
Market Challenges: High cost of treatment
Data security and privacy concerns around the use and sharing of sensitive patient information can hamper the global pharmaceutical manufacturing software market growth. The pharmaceutical industry deals with highly confidential data related to intellectual property, clinical trials, and patient health records. Any breach of this data can lead to legal liabilities, loss of patient trust, and reputational damage. With pharmaceutical manufacturing software deployed across global supply chains, there are several touchpoints where patient data is stored, processed, and transferred. This extensive data footprint increases the risk of a security lapse. Databases containing formulations, testing results, and commercial contracts are also targeted by cybercriminals for financial gain. Manufacturers struggle to ensure compliance with privacy laws in all jurisdictions while sharing information internationally. Additional requirements to anonymize and encrypt sensitive fields add costs and complexity to digital projects.
Market Opportunities: Adoption of AI and IoT based solutions
The pharmaceutical industry has been adopting emerging technologies like artificial intelligence and Internet of Things to make manufacturing processes more efficient and optimize production. AI and machine learning algorithms can analyze massive amounts of data from different stages of production to detect anomalies and predict failures or defects. This enables pre-emptive corrections and reduces downtime. IoT-connected sensors can remotely monitor manufacturing equipment almost in real-time for parameters like temperature, pressure and vibration levels. Any deviations can immediately trigger alerts to engineers, who can take necessary corrective actions remotely without needing to visits plant sites. This round-the-clock monitoring improves compliance with regulatory standards and allows for more consistent production quality control.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
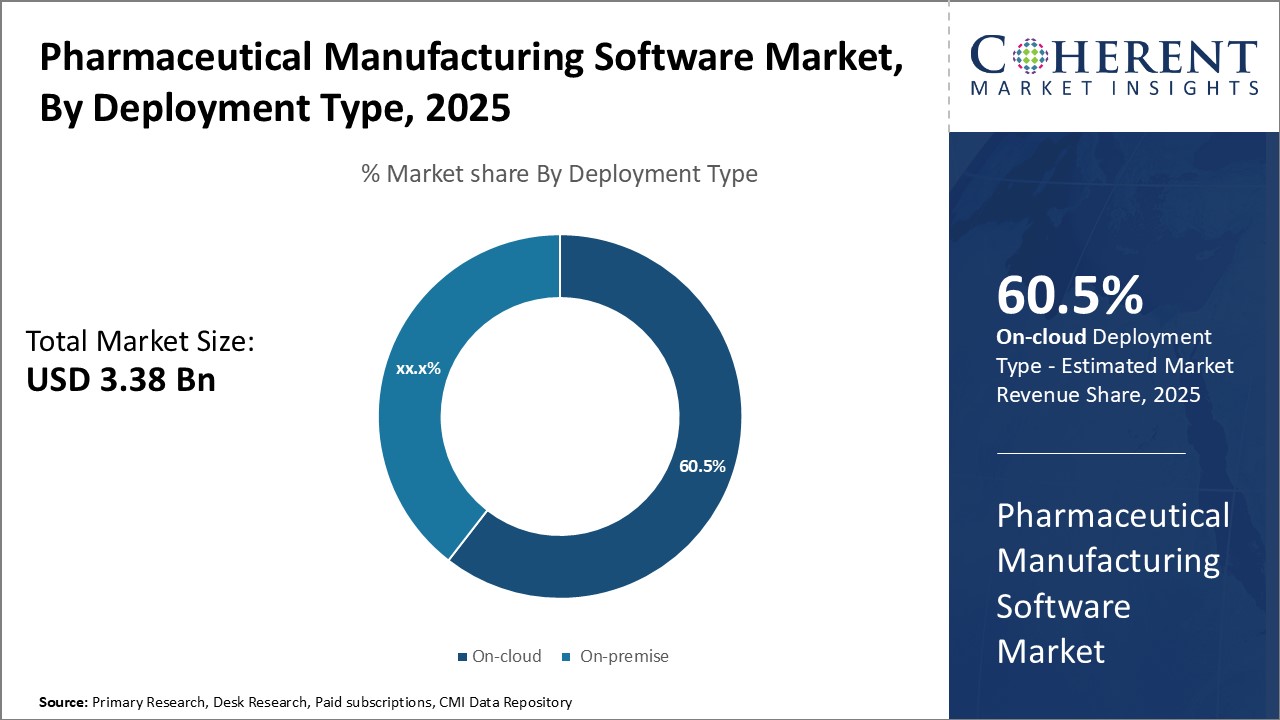
Insights, By Deployment Type - Cloud convenience boosts adoption of on-cloud
In terms of deployment type, on-cloud segment is estimated to contribute the highest market share of 60.5% in 2025, owing to the numerous advantages it offers over the on-premise deployment type. On-cloud or SaaS model allows pharmaceutical companies to access manufacturing software from anywhere without needing to install it locally. This enables flexible, distributed working styles and offers instant scalability. The on-cloud deployment is also less expensive as compared to on-premise as there is no requirement for huge upfront capital expenditure on hardware and related infrastructure. With a simple subscription-based pricing, companies can get access to powerful software tools without straining their budgets. The on-cloud deployment provides significant simplification of IT management burdens for pharmaceutical firms. Hosted off-site by experts, the on-cloud software requires minimal in-house maintenance and upgrades. This frees up internal IT staff to focus more on core tasks. On-cloud software seamlessly integrates with other cloud-based tools that companies already use for various functions. This allows smoother interoperability and data sharing across different departments. The automatic software updates and added security features that come with on-cloud also help ensure that regulatory compliances are met.
Insights, By Application- Compliance management drives uptake in large enterprises
In terms of application, large enterprises segment is estimated to contribute the highest market share of 60.12 % in 2025, due to their extensive regulatory obligations. Stringent requirements around quality management, serialization, batch processing, and reporting place a huge onus on large pharmaceutical firms to streamline compliance processes. Manufacturing software optimizes regulatory adherence through features like electronic batch records, smart deviation handling, and serialization. These also facilitate automation of critical compliance workflows to boost efficiency. Given their high volumes and complex nature of operations across multiple sites, large companies have a massive scale of record-keeping and reporting needs. Manufacturing software offers centralized data management and powerful analytics capabilities to simplify governance tasks. Their sophisticated quality management modules help incorporate best practices, standardize operations worldwide, and ensure product quality is consistent through every stage. This assumes high importance considering large firms cater to a huge patient base.
Insights, By End User- R&D automation boosts demand among biopharmaceutical companies
In terms of end user, biopharmaceutical companies segment is estimated to contribute the highest market share of 30.12 % in 2024, due to their pressing need to streamline complex R&D functions. From drug discovery to clinical trials, every stage in the biopharma value chain relies on intensive experimentation and analysis of massive data pools. However, manual methods struggle to keep pace with the speed of scientific innovation, thus, this makes automating drug development workflows through specialized software highly important. Manufacturing software offers powerful laboratory automation modules to optimize biomanufacturing processes. Features like electronic lab notebooks, smart inventory management, and LIMS integration help biopharma firms run collaborative multi-site research in a compliant manner. These facilitate faster analysis of pre-clinical findings and accelerate molecule screening procedures. Integrated ELN capabilities also ensure seamless data archival of all intellectual property. This assumes significance considering the huge R&D investments of biopharma companies.
Need a Different Region or Segment? Download Free Sample
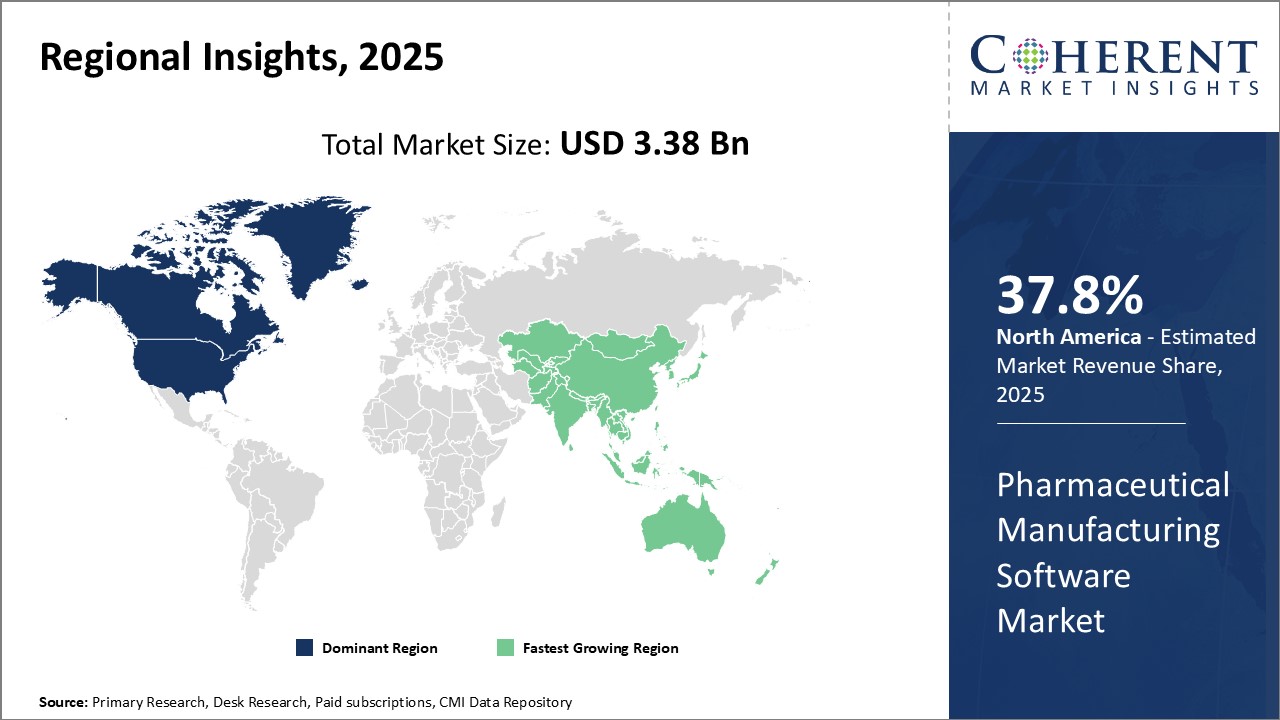
North America is expected to dominate the pharmaceutical manufacturing software market with an estimated market share of 37.8% in 2025. The region is home to leading pharmaceutical companies that have been investing heavily in advanced software technologies to drive efficiency and optimize production processes. The strict regulatory norms laid out by agencies such as the FDA have compelled drug manufacturers to rely on sophisticated software solutions to ensure compliance. Several global pharmaceutical giants also have their headquarters located in the U.S. and Canada, allowing them to benefit from proximity to software developers based in the region. Furthermore, North America boasts a higher per capita healthcare spending compared to other regions, empowering pharmaceutical companies to flex their financial muscles on process automation technologies.
Asia Pacific region is emerging as the fastest growing market for pharmaceutical manufacturing software. Countries like China, India and Japan has large concentration of generic drug manufacturers that focuses on quality and timely delivery. While these companies traditionally lagged in digitization, the need to streamline production and cut costs are compelling them to follow regulatory counterparts in developed markets towards software-enabled operations. Asia Pacific also serves as the manufacturing hub for several Western pharmaceutical companies due to a lower cost of production. This factor is substantiating foreign investment into upgrading legacy infrastructure with advanced digital technologies. Rapidly developing economies and growing income levels boosts higher healthcare consumption in Asia Pacific, with domestic companies striving to meet the region's needs as a competitive advantage.
Pharmaceutical Manufacturing Software Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 3.38 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.0% | 2032 Value Projection: | USD 6.18 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
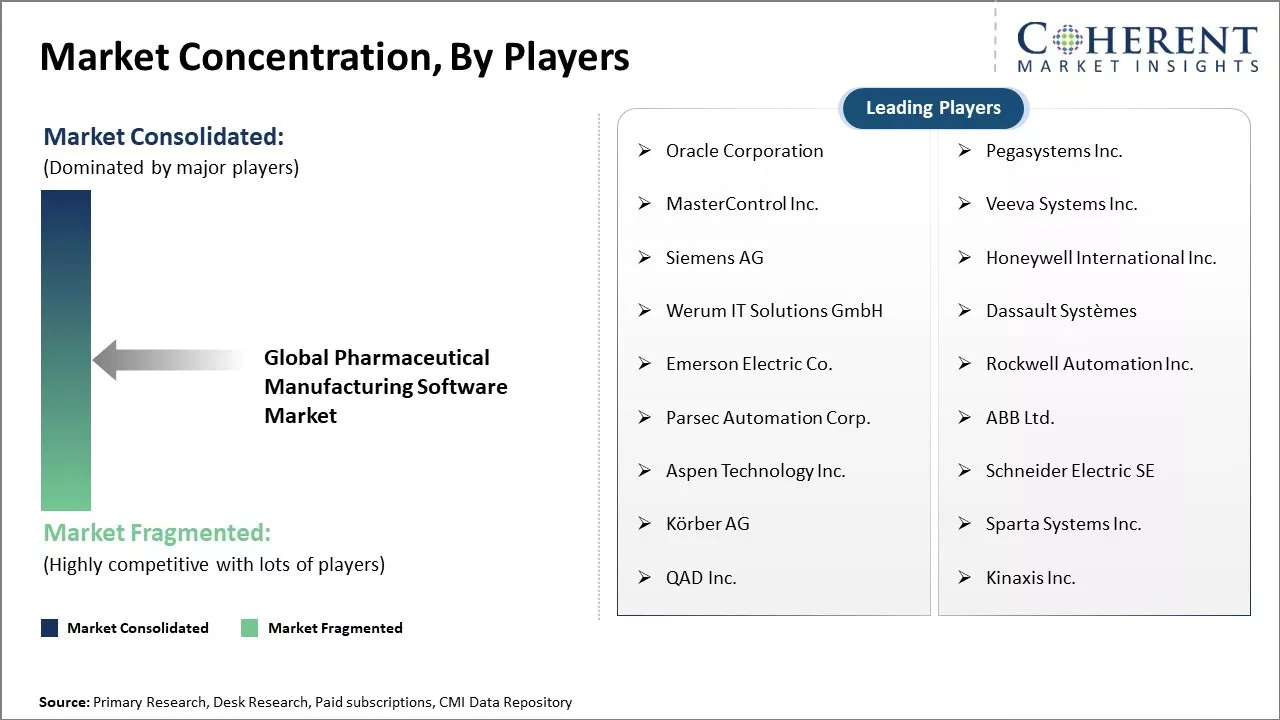
Oracle Corporation, Pegasystems Inc., MasterControl Inc., Veeva Systems Inc., Siemens AG, Honeywell International Inc., Werum IT Solutions GmbH, Dassault Systèmes, Emerson Electric Co., Rockwell Automation Inc., Parsec Automation Corp., ABB Ltd., Aspen Technology Inc., Schneider Electric SE, Körber AG, Sparta Systems Inc., QAD Inc., Kinaxis Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients