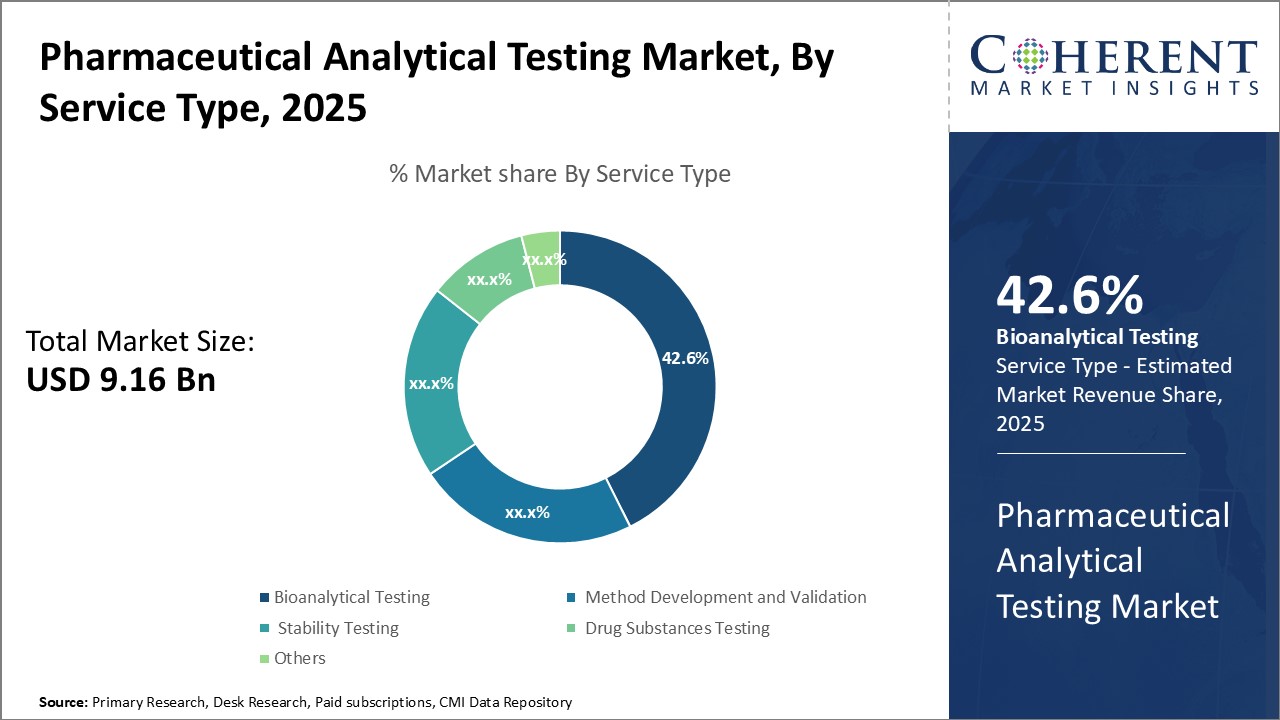
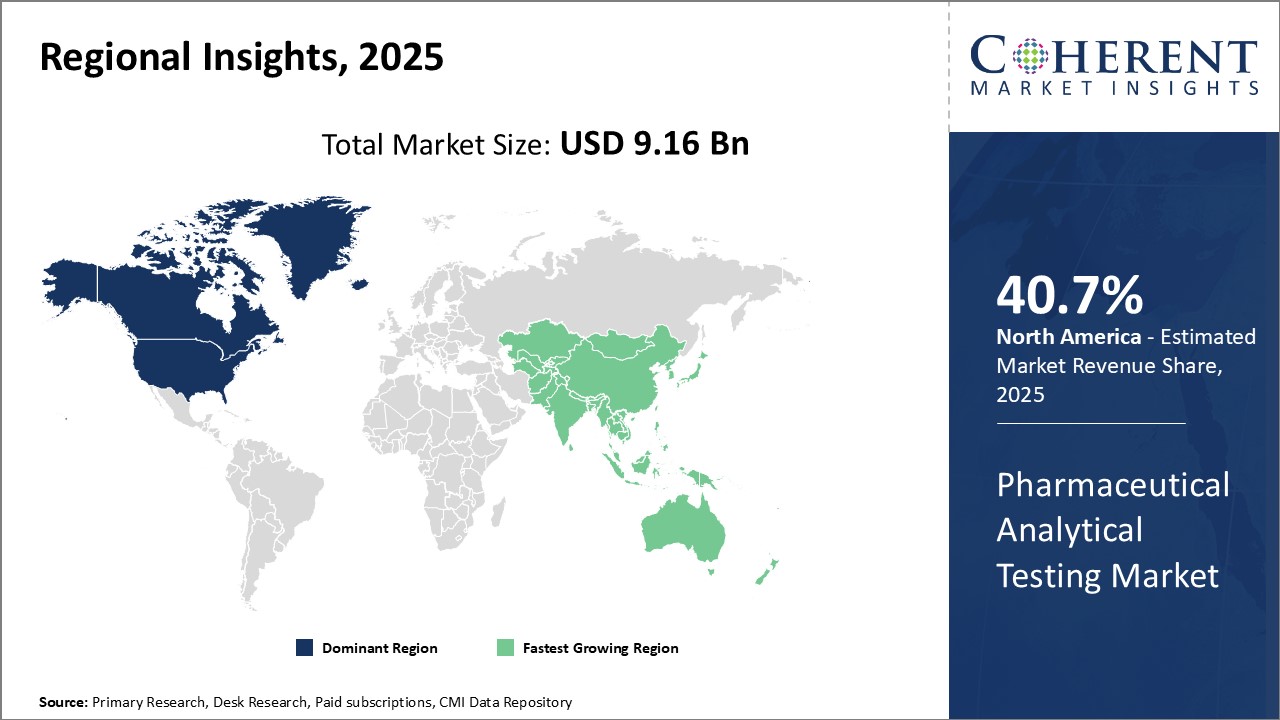
The pharmaceutical analytical testing market is estimated to be valued at USD 9.16 billion in 2025 and is expected to reach USD 16.65 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 8.9% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
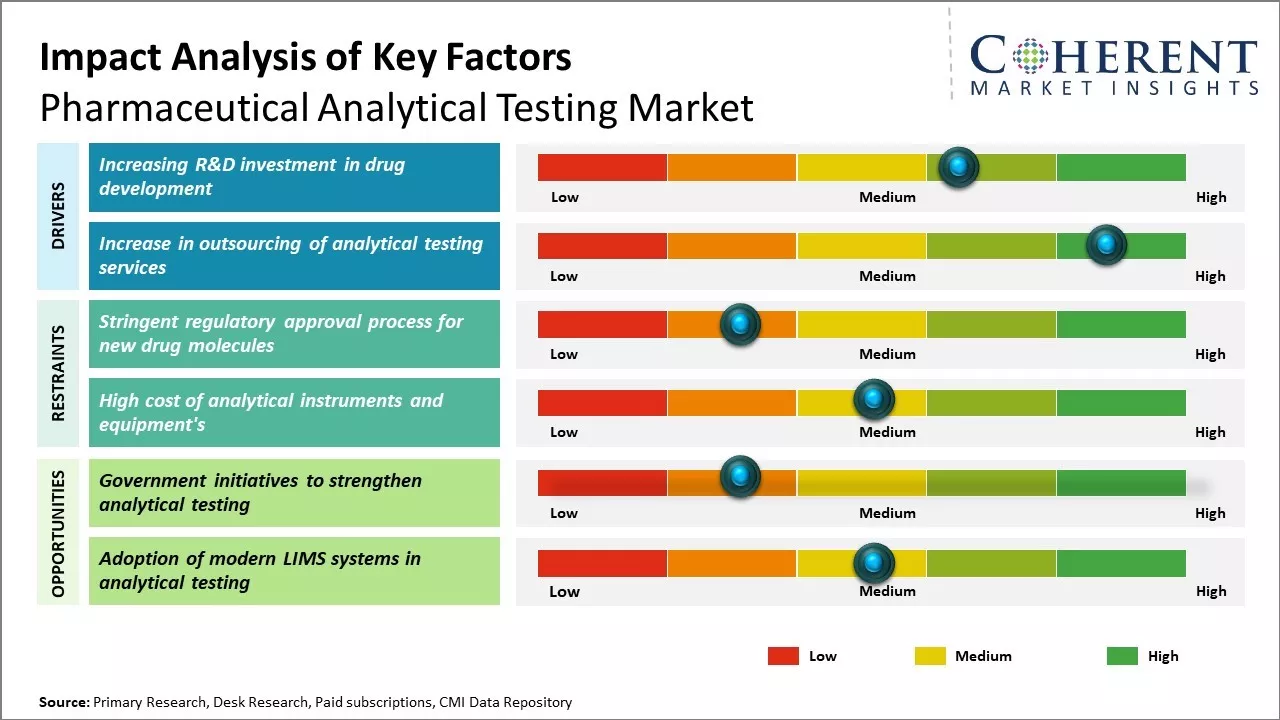
The pharmaceutical analytical testing market is driven by the increased R&D expenditure in pharmaceutical and biotechnology companies. The stringent regulatory guidelines for drug development and safety have increased the demand for analytical testing. Moreover, the growth of generics and biosimilars has significantly contributed to the market expansion. However, the high cost of analytical instruments and lack of skilled professionals are the major factors limiting the market growth.
Increasing R&D investment in drug development
Increased research and development activities, along with collaborations and strategic agreements, are expected to fuel the market growth. For instance, in November 2021, Alcami Corporation, Inc., a contract development and manufacturing organization, entered into a master laboratory services agreement with Novavax, a biotechnology company. Through this agreement, Novavax secured full-time equivalent (FTE) resources to provide analytical testing support for its recombinant nanoparticle protein-based COVID-19 vaccine candidate with Matrix-M adjuvant. These developments are projected to drive market expansion.
Get actionable strategies to beat competition: Download Free Sample
Increase in outsourcing of analytical testing services
The pharmaceutical analytical testing market is poised for growth over the forecast period due to the rising global demand for analytical testing services and solutions. For example, bio-pharmaceutical companies utilize analytical testing to characterize biologics and biosimilars, enhance productivity, and ensure real-time product quality control. In July 2021, LGM Pharma, a pharmaceutical outsourcing company, introduced its Analytical Services offering, providing analytical testing and stability services to pharmaceutical industry clients. LGM Pharma now extends its analytical services expertise and facilities as a standalone contract service for pharmaceutical industry clients. Furthermore, the surge in drug approvals and clinical trials worldwide is anticipated to bolster the expansion of the global pharmaceutical analytical testing market. Notably, the number of registered clinical trials has notably increased in recent years. Despite the ongoing impact of COVID-19, the U.S FDA's approval count remained consistent with recent trends last year. In 2021, the FDA's Center for Drug Evaluation and Research (CDER) greenlit 50 novel therapeutics, aligning with the five-year average of 51 drugs per year. A decade ago, the average was 24 drugs per year. This trend is expected to drive market growth.
Key Takeaways of Analyst:
The pharmaceutical analytical testing market is expected to grow steadily driven by the increasing complexity of drug formulations and the stringent regulatory environment. The need for thorough quality control and safety testing during drug production will drive demand for sophisticated analytical testing solutions. North America will continue to dominate due to established pharmaceutical industries and strict quality standards mandated by regulatory bodies. However, Asia Pacific is likely to experience the fastest growth with expanding generic drug manufacturing in countries like India and China. A potential restraint could be the high costs associated with cGMP-compliant analytical testing equipment and infrastructure. This may act as a barrier for small players and drug makers in emerging markets. Further consolidation across analytical instrument vendors may lead to higher prices of analytical solutions in the long run. On the other hand, outsourcing of testing services to specialized CROs presents an opportunity for contract testing laboratories to see higher revenues. Growth of biologics and personalized medicine also opens up opportunities for analytical players to develop specialized solutions for large molecule characterization.
Market Challenges: Stringent regulatory approval process for new drug molecules
Stringent regulations and compliance standards set by global regulatory bodies have increased the compliance burden for companies. Additionally, the cost of analytical method development and validation combined with pressure to reduce drug development timelines and costs has made it difficult for labs to scale operations profitably. Finding skilled analytical chemists and maintaining aging testing equipment also impacts market players. Adopting newer technologies like artificial intelligence and automation comes with associated training and investment costs.
Market Opportunities: Government initiatives to strengthen analytical testing
Government initiatives to enhance analytical testing capabilities offer growth opportunities in the pharmaceutical analytical testing market. For instance, according to the WHO, around 349 vaccines were in development as of April 15, 2022. This is anticipated to drive up the demand for analytical testing services for COVID-19 vaccines that are undergoing clinical trials. Johnson & Johnson tested Ad26.COV2.S, an investigational COVID-19 vaccine candidate, with support from Eurofins Scientific.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
Insights, By Service Type: Bioanalytical testing contributes the highest share of the market owing to increasing R&D investment in biologics and biosimilars
The bioanalytical testing segment holds the largest share which is 42.6% in the pharmaceutical analytical testing market due to growing investment in biologics and biosimilars development. Bioanalytical testing involves quantitative and qualitative analysis of molecules in biological matrices to support pharmacokinetic and bioequivalence studies. With many blockbuster drugs going off-patent, pharmaceutical companies are focusing on developing complex biologics drugs such as monoclonal antibodies, recombinant proteins, and stem cell and gene therapy products. Strict regulatory guidelines around efficacy, safety, and quality testing of these biotherapeutics is also propelling demand for specialized bioanalytical testing services. Additionally, biosimilars market is expected to witness high growth in the coming years. Determining pharmacokinetic similarity between a biosimilar and its reference product requires robust bioanalytical methods and bioanalysis, which contributes to the leading position of this segment. Stringent regulatory oversight and increasing investment in biosimilars development will likely continue driving the need for high quality bioanalytical testing services.
Insights, By End User: Biopharmaceutical companies contribute the highest share of the market owing to their extensive investment in drug development and clinical trials
Biopharmaceutical companies dominate the pharmaceutical analytical testing market as end users due to their large R&D budgets and emphasis on drug innovation with 44.62% of the market share. These companies extensively utilize analytical testing at various stages including method development and validation, stability testing, quality control, and bioequivalence studies to support preclinical and clinical development of new molecular entities. Particularly, outsourcing analytical testing requirements helps these companies to reduce capital investment and access advanced technologies. The complex nature of biologics and biosimilars under development also inflates analytical testing needs of biopharma players. Additionally, stringent regulatory changes regarding pharmaceutical quality necessitates continuous analytical testing of manufactures products post approval as well. Overall, growing R&D expenditure of biopharma giants on novel drug development and clinical trials make them the primary consumer of pharmaceutical analytical testing services worldwide.
Need a Different Region or Segment? Download Free Sample
North America has established itself as the dominant region in the global pharmaceutical analytical testing market with the highest share of 40.7%. The U.S., in particular, accounts for the largest share due to a presence of many major pharmaceutical companies and contract research organizations in the country. Also supporting the market growth is the well-established regulatory guidelines mandating quality control and stringent analytical testing of drugs. This ensures pharmaceutical companies dedicate sizable investments in developing state-of-the-art testing infrastructure and hiring top analytical chemists and scientists. Furthermore, the U.S. FDA regularly inspects pharmaceutical manufacturing facilities and approves new drug applications based on comprehensive analytical reports. This has made pharmaceutical analytical testing a significant part of the drug development and approval process.
The Asia Pacific region has emerged as the fastest growing market for pharmaceutical analytical testing over the past decade. Countries such as India, China, Japan, and South Korea have become global manufacturing hubs for pharmaceutical formulations as well as active pharmaceutical ingredients. This has led to a robust demand for outsourced analytical testing services from both domestic as well as multinational drug companies. The region also attracts considerable investments from major pharmaceutical firms seeking to establish local manufacturing bases and R&D centers. This helps generate additional requirement for analytical methodology development, validation testing services, and routine quality control support. The regulatory mandates across Asia Pacific nations are also progressively becoming more stringent. This translates to higher compliance standards for manufactures and increased dependency on analytical testing providers. The large talent pool of analytical chemists and relatively lower operating costs in some Asia Pacific countries are additional factors driving growth. Lower pricing for testing services allows pharmaceutical companies to gain competitive advantage.
Pharmaceutical Analytical Testing Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 9.16 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.9% | 2032 Value Projection: | USD 16.65 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
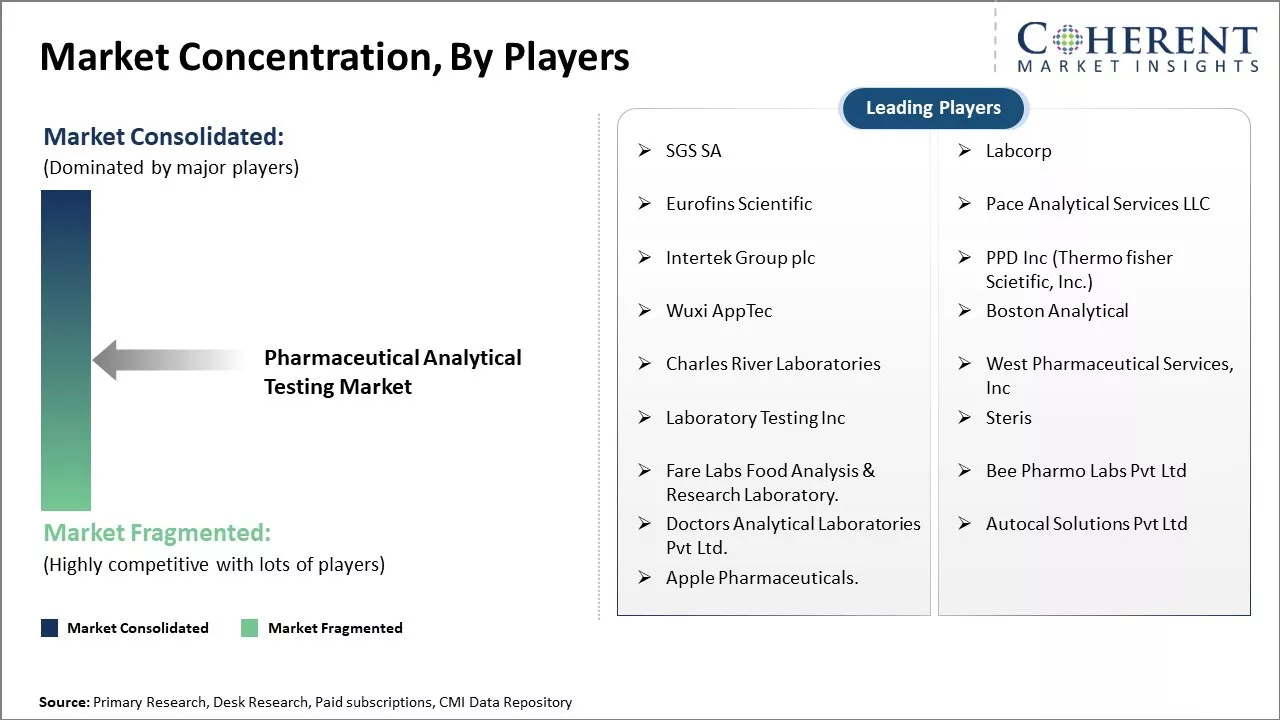
| Companies covered: |
SGS SA, Labcorp, Eurofins Scientific, Pace Analytical Services LLC, Intertek Group plc, PPD Inc (Thermo fisher Scietific, Inc.), Wuxi AppTec, Boston Analytical, Charles River Laboratories, West Pharmaceutical Services, Inc, Laboratory Testing Inc, Steris, Fare Labs Food Analysis & Research Laboratory. , Bee Pharmo Labs Pvt Ltd, Doctors Analytical Laboratories Pvt Ltd., Autocal Solutions Pvt Ltd, and Apple Pharmaceuticals. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: Pharmaceutical analytical testing refers to a range of laboratory tests performed on pharmaceutical drug products and substances at various stages of the development and manufacturing processes to ensure safety, efficacy, and consistency. Tests include identity, purity and impurities analysis using techniques such as chromatography, spectroscopy, and dissolution. The results must comply with tight specifications mandated by regulatory authorities to assure quality standards are maintained and finished drug products released for clinical trials and market distribution meet all requirements. Pharmaceutical analytical testing plays a key role in drug development and production.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients