Peyronies Disease Treatment Market Size and Forecast – 2026 – 2033
The Global Peyronies Disease Treatment Market size is estimated to be valued at USD 1.15 billion in 2026 and is expected to reach USD 2.35 billion by 2033, exhibiting a compound annual growth rate (CAGR) of 10.8% from 2026 to 2033.
Global Peyronies Disease Treatment Market Overview
Products in the Peyronie’s disease treatment market include pharmaceuticals, medical devices, and minimally invasive therapies designed to reduce penile curvature and alleviate pain associated with Peyronie’s disease. Treatment options range from injectable drugs (such as collagenase clostridium histolyticum) that break down scar tissue to penile traction devices and shockwave therapy systems that promote tissue remodeling. Surgical prostheses and tunical plication devices are used for severe cases. These products aim to improve sexual function, reduce deformity, and enhance patient quality of life. They are typically used by urologists and sexual health specialists.
Key Takeaways
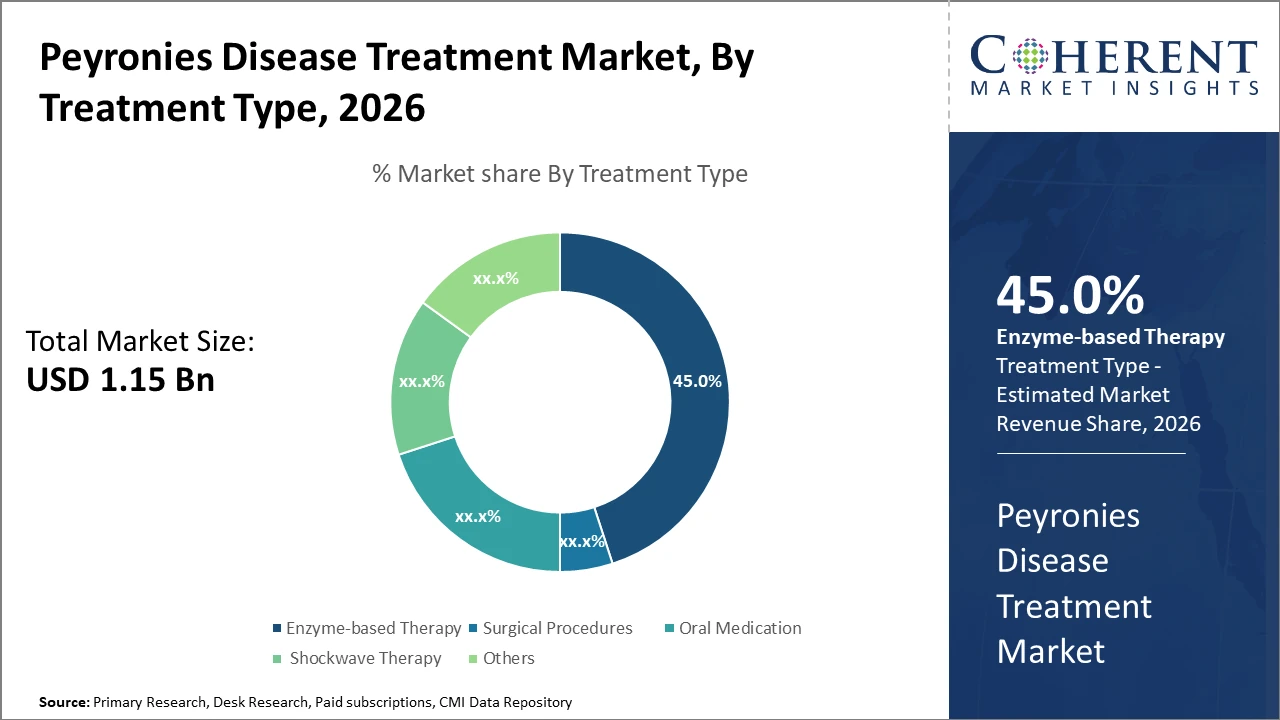
The enzyme-based therapy segment dominates the Peyronies Disease Treatment market due to its clinical effectiveness and patient preference, accounting for approximately 45% market share. Oral medications and surgical procedures are also gaining traction as part of market diversification strategies.
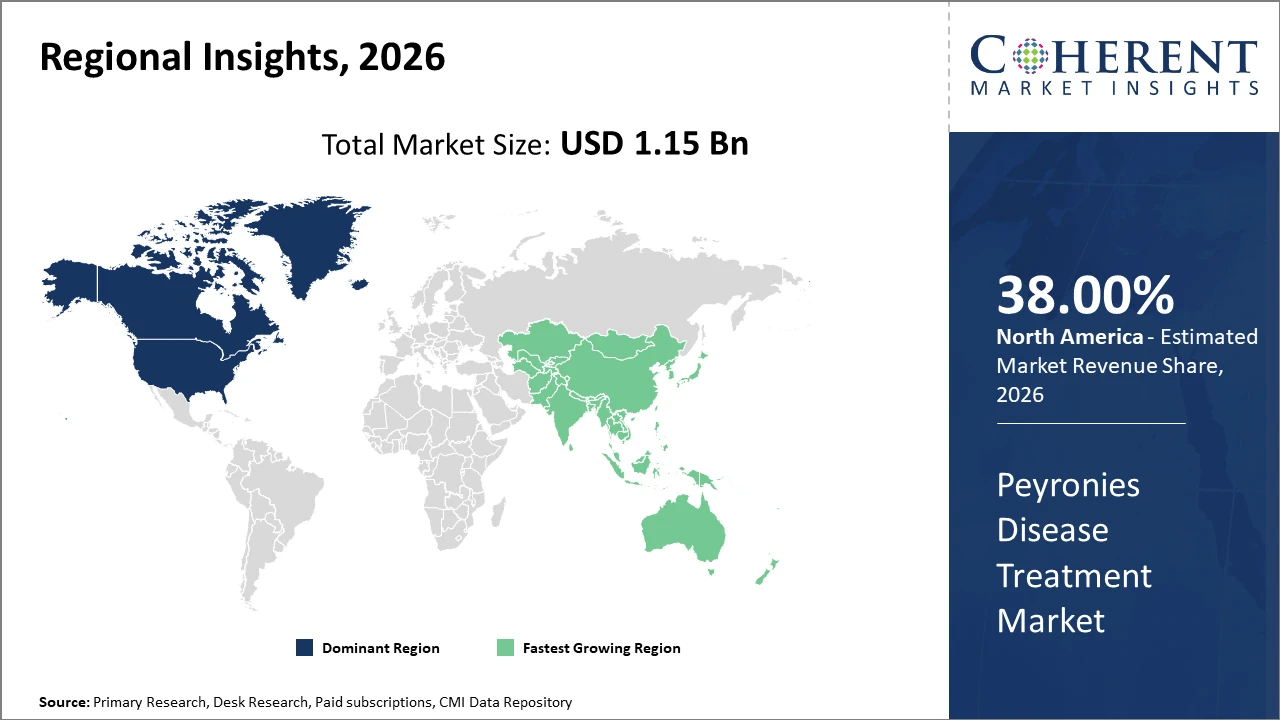
North America commands a major industry share by 38% in the Peyronies Disease Treatment market, driven by advanced healthcare facilities and ongoing clinical research. Europe follows with impactful regulatory initiatives promoting innovative therapies.
Asia Pacific is witnessing the fastest market growth attributed to increasing awareness programs and expanding healthcare access, projecting substantial business growth opportunities for market companies investing in the region.
Market trends reflect a shift towards integrated treatment regimens and digital diagnostic tools, which are expected to redefine future market analysis and revenue models.
Peyronies Disease Treatment Market Segmentation Analysis
To learn more about this report, Download Free Sample
Peyronies Disease Treatment Market Insights, By Treatment Type
Enzyme-based therapy dominates the market share, capturing nearly 45% due to the demonstrated effectiveness of collagenase injections in non-surgical management. This segment's growth is propelled by increasing clinical evidence supporting enzyme therapies, as well as patient preference for less invasive treatments. Surgical procedures remain a critical segment, especially for advanced cases, though it holds a lower market share due to the emergence of less invasive options. Oral medications and shockwave therapy are gaining traction as complementary or alternative therapies, with steady adoption rates in outpatient care settings.
Peyronies Disease Treatment Market Insights, By End-User
Hospitals dominate the market share due to their comprehensive infrastructure and broad patient base, handling complex cases requiring surgical interventions alongside pharmacological treatments. Specialty clinics representing urology-focused outpatient services are the fastest-growing subsegment, fueled by increased disease awareness and preference for targeted care solutions. Additionally, ambulatory surgical centers are notable for providing cost-efficient procedural treatments with reduced hospital stays, gaining ground especially in North America and Europe.
Peyronies Disease Treatment Market Insights, By Distribution Channel
Hospital pharmacies hold the dominating market share, primarily attributable to direct supply chains supporting inpatient and outpatient therapy administrations. Online pharmacies are the fastest-growing channel segment, driven by expanding e-commerce adoption, especially in developed markets where digital health infrastructure is mature. Retail pharmacies continue to play a significant role in providing oral medications and supportive therapies, particularly in emerging regions. The ‘others’ segment includes specialty distributors and government healthcare supply programs facilitating broader access.
Peyronies Disease Treatment Market Trends
Recent market trends underscore the growing significance of biologic enzyme-based therapies, specifically the success of collagenase clostridium histolyticum in reducing surgical intervention rates by 18% in 2025.
Additionally, telemedicine platforms have influenced early diagnosis and treatment adherence, with virtual consultations increasing by 30% since 2024.
The shift from invasive surgeries to minimally invasive and pharmacologic treatments signifies a critical evolution in market dynamics, encouraging further investment into research.
Peyronies Disease Treatment Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Peyronies Disease Treatment Market Analysis and Trends
In North America, the dominance in the Peyronies Disease Treatment market is driven by a mature healthcare ecosystem, presence of multiple market players, and favorable reimbursement policies. U.S. healthcare expenditure focused on urological disorders increased by 6.7% in 2025, supporting market growth. Notable companies headquartered in this region lead clinical trials and innovation pipelines, bolstering industry share.
Asia Pacific Peyronies Disease Treatment Market Analysis and Trends
Meanwhile, Asia Pacific exhibits the fastest growth with a CAGR exceeding 14%, fueled by rising awareness campaigns, expanding hospital infrastructure, and increasing healthcare spending, especially in China and India. Evolving regulatory frameworks promoting drug approvals and medical device import facilitation have accelerated business growth, supported by collaborations between local and international market players.
Peyronies Disease Treatment Market Outlook for Key Countries
USA Peyronies Disease Treatment Market Analysis and Trends
The USA’s Peyronies Disease Treatment market experienced dynamic growth driven by technological innovation and widespread adoption of enzyme-based therapies. In 2025, over 60% of patients undergoing treatment opted for FDA-approved therapies like CCH, complemented by increasing investment in outpatient clinics specializing in urology. The country’s advanced healthcare infrastructure and supportive reimbursement policies have further propelled market companies to launch next-generation minimally invasive surgical devices. Key players such as Auxilium Pharmaceuticals and Boston Scientific dominate the landscape by introducing streamlined clinical care models and expanding education programs for healthcare providers.
Germany Peyronies Disease Treatment Market Analysis and Trends
Germany’s market benefits from a strong healthcare framework and increasing public awareness initiatives, which led to a 12% rise in Peyronie’s disease diagnoses in 2025 compared to previous years. The country’s health insurance programs facilitate access to both surgical and pharmaceutical therapies, boosting market revenue. Local companies like Coloplast, along with multinational corporations, have focused on developing combination therapy protocols. Collaborative research initiatives between universities and industry players further enhance technological advancements within the German Peyronies Disease Treatment ecosystem.
Analyst Opinion
Increasing adoption of enzyme-based therapies is a key quantitative indicator driving the market share for Peyronie’s Disease Treatment. For instance, collagenase clostridium histolyticum (CCH) recorded over 120,000 treatment cycles in 2025 alone, reflecting a stronger demand trajectory. Pricing strategies by pharmaceutical companies have also aligned to improve patient access, with average treatment costs decreasing by 7% from 2024 to 2026, thus accelerating market expansion.
The demand-side indicators show notable growth driven by increased diagnostics and early intervention. Data from urology clinics in the U.S. suggest a 15% rise in Peyronie’s diagnoses year-over-year since 2024, advocating increased market opportunity for therapeutic interventions. Additionally, imports of innovative medical devices used in minimally invasive surgeries surged by 18% in North America in 2025, indicating readiness for advanced treatment options.
Micro-indicators at the molecular therapeutic level are emerging, particularly in clinical trial activities. More than 35 new molecular entities for Peyronie’s treatment entered trials from 2024 to 2026, underscoring the ongoing pipeline developments that will influence future market share. Production capacity for targeted oral and injectable drug forms expanded by 22% globally in 2025, ensuring supply-side alignment with increasing demand.
Regional consumption data reveal that Asia Pacific’s Peyronies Disease Treatment market revenue grew by 14.5% in 2025, fueled by increased healthcare expenditure and government-led screening programs. This regional demand dynamics alongside favorable regulatory frameworks is reshaping global market growth strategies, with new market players entering via joint ventures and licensing agreements.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 1.15 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 10.8% | 2033 Value Projection: | USD 2.35 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Jive Pharmaceuticals, Accelerated Medical Development, Novartis AG, Sun Pharmaceutical Industries, Zydus Cadila, Biocon Limited, Allergan plc, Meda Pharma, GlaxoSmithKline, Bayer AG | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Peyronies Disease Treatment Market Growth Factors
The expanding prevalence of Peyronie’s disease, estimated to affect nearly 1 in 10 men over 40 years of age, is a core market driver. Emerging pharmacological treatments providing improved efficacy with minimal invasiveness are gaining traction, demonstrated by a 20% increase in CCH utilization globally in 2025. Enhanced reimbursement frameworks in developed regions are facilitating patient access to advanced therapies, accounting for a 15% rise in treatment uptake in 2026. Additionally, increased investment in R&D focused on novel biologics and regenerative medicine offers promising growth stimuli, with over USD 50 million allocated to clinical trials in 2025 alone. These factors collectively underpin consistent business growth and market revenue amplification.
Peyronies Disease Treatment Market Development
In 2024, Endo International, in collaboration with the Sexual Medicine Society of North America (SMSNA), launched a Peyronie’s Disease Self-Assessment App as a digital health initiative to help men discreetly track symptoms, monitor disease progression, and recognize when to seek medical care. The app is designed to promote earlier diagnosis and timely intervention by increasing awareness and patient engagement in the management of Peyronie’s disease.
Key Players
Leading Companies of the Market
Jive Pharmaceuticals
Accelerated Medical Development
Sun Pharmaceutical Industries
Zydus Cadila
Biocon Limited
Allergan plc
Meda Pharma
GlaxoSmithKline
Bayer AG
Leading companies have increasingly adopted market growth strategies centered around mergers and acquisitions to expand their treatment portfolios. For example, Ipsen's acquisition of Auxilium Pharmaceuticals in 2024 broadened its access to Peyronie’s treatment formulations, resulting in a 15% increase in market share in North America. Similarly, strategic partnerships between pharmaceutical companies and biotechnology firms have accelerated innovative therapy pipelines, as evidenced by Boston Scientific’s collaboration for drug delivery systems in 2025, enhancing their competitive positioning.
Peyronies Disease Treatment Market Future Outlook
The future of Peyronie’s disease treatment is likely to be shaped by ongoing efforts to improve both efficacy and patient experience. Advances in biologics and localized drug delivery systems may yield more targeted therapies with fewer systemic effects. Combination therapies that integrate pharmacologic and device-based approaches have the potential to improve outcomes, particularly for patients with early-stage disease. Surgical techniques will continue to refine less invasive options with faster recovery and better functional results, supported by improvements in imaging and planning tools. Increased understanding of the molecular mechanisms underlying fibrosis may open avenues for preventive strategies or therapies that reverse disease progression. As awareness of Peyronie’s disease grows among both clinicians and patients, and as quality-of-life outcomes become central metrics in evaluation, treatment paradigms are expected to broaden, offering more personalized and effective care.
Peyronies Disease Treatment Market Historical Analysis
The Peyronie’s disease treatment market historically faced challenges due to the complex pathophysiology of the condition, which involves the development of fibrous scar tissue within the penile shaft, leading to curvature, pain, and erectile dysfunction. Initial treatment approaches were primarily conservative, including oral medications and physical therapy techniques that often yielded limited efficacy. Surgical intervention was once considered the gold standard for severe deformities, but it carried risks of complications and extended recovery times.
In the early 2000s, the market experienced a paradigm shift with the development and approval of injectable collagenase clostridium histolyticum, which specifically targeted the plaque formation in affected tissue. This non-surgical option provided a mechanism-based treatment that expanded therapeutic possibilities. Over time, increased clinical research into traction therapy devices and shockwave therapy systems contributed additional, albeit adjunctive, treatment modalities, though adoption remained uneven due to variance in clinical outcomes and patient compliance.
Sources
Primary Research Interviews:
Urologists
sexual health specialists
medical device manufacturers
hospital procurement managers
clinical researchers
Databases:
WHO Urology Data
FDA Drug Approvals
ClinicalTrials.gov
OECD Health Statistics
Magazines:
Urology Times
Medical Device Network
Men’s Health (Clinical)
Healthcare Weekly
MedTech Insight
Journals:
Journal of Urology
Sexual Medicine Reviews
International Journal of Impotence Research
BJU International
Andrology
Newspapers:
Reuters Health
Financial Times (Healthcare)
The Guardian (Health)
The New York Times (Medicine)
Bloomberg Health
Associations:
American Urological Association
European Association of Urology
Sexual Medicine Society of North America
WHO
International Society for Sexual Medicine
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients