Peripherally Inserted Central Catheters Market is estimated to be valued at USD 1,117.6 Mn in 2025 and is expected to reach USD 1,782.9 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 6.9% from 2025 to 2032.
Analysts’ views on global peripherally inserted central catheters market:
Increasing prevalence of these diseases increases the need of chemotherapy treatment which uses peripherally inserted central catheters (PICC) and ultimately shows positive impact on global peripherally inserted central catheters market. For instance, according to the data published by the World Health Organization, on February 03, 2022, cancer is a leading cause of death worldwide, accounting for nearly 10 million deaths in 2020, or nearly one in six deaths. Breast and lung cancers were the most common cancers worldwide, contributing 12.5% and 12.2% of the total number of new cases diagnosed in 2020. Colorectal cancer was the third most common cancer with 1.9 million new cases in 2020, contributing 10.7% of new cases.
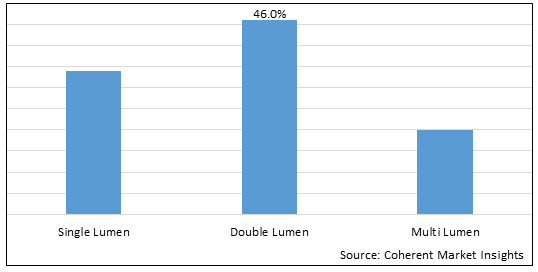
Figure 1. Global Peripherally Inserted Central Catheters Market Share (%), By Product Type, 2025
To learn more about this report, Download Free Sample
Global Peripherally Inserted Central Catheters Market– Drivers
Increasing prevalence of infectious disease
Increasing prevalence of infectious disease is expected to drive the global peripherally inserted central catheters market growth over the forecast period. For instance, according to the data shared by the Centers for Disease Control and Prevention, on January 25, 2023, worldwide burden of infectious diseases has increased from 37.9% to 61.8%. High-income nations like Italy, Japan, Singapore, and Canada typically account for more than 80 % of disease burden. In U.S. in 2020, the number of new cases of tuberculosis (8,916), salmonella (58,371), Lyme disease (34,945), and meningococcal disease (371) were rising constantly. Such infectious diseases require antibiotics therapy that uses peripherally inserted central catheters (PICC). This ultimately shows positive impact on global peripherally inserted central catheters market.
Increasing Launches of New Technologies & Products
Increasing launch of new products to make the working process of catheters more efficient is expected to drive the global peripherally inserted central catheters market growth. For instance, on February 2, 2022, Zeus Company Inc., a Singapore-based precision polymer manufacturer announced that it has added Polytetrafluoroethylene (PTFE) Sub-Lite-Wall multi-lumen tubing to its product portfolio. Zeus Company Inc.’s PTFE Sub-Lite-Wall multi-lumens have average max wall thicknesses ranging from 0.002" to 0.005" (0.051 mm to 0.127 mm). In addition to ultra-thin walls, the new product features high structural integrity, improved planarity, high lubricity, and excellent dielectric strength. It is biocompatible (certified United States Pharmacopeia (USP) Class VI) and has a working temperature of 260 °C (500 °F).
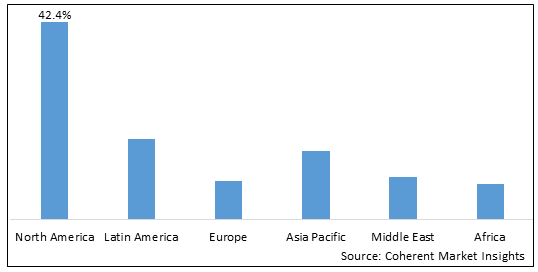
Figure 2 Global Peripherally Inserted Central Catheters Market Share (%), By Region, 2025
To learn more about this report, Download Free Sample
Global Peripherally Inserted Central Catheters Market - Regional Analysis
Among region, North America is estimated to hold a dominant position in the global peripherally inserted central catheters market over the forecast period, owing to increasing approval of products. For instance, on April 30, 2020, Biowy Corporation, a U.S.-based medical devices & equipment manufacturing company, announced that it has received U.S. Food and Drug Administration 510(k) clearance for its Biowy PICC Catheter S Kit.
Global Peripherally Inserted Central Catheters Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries, such as China, India, Saudi Arabia, the U.A.E., Egypt, and others faced problems regarding transportation of drugs from one place to another.
However, the COVID-19 pandemic had negative impact on the global peripherally inserted central catheters market, due to reduced healthcare facilities during lockdown period. For instance, according to an article published by the Antimicrobial Resistance & Infection Control, on June 4, 2021, COVID-19 has caused reduced the attention to traditional healthcare-associated infections (HAI) prevention programs in terms of lack of surveillance efforts, process measures, and containment strategies. Supply shortages of personal protective equipment’s, and less disposable income during COVID-19 also impacted negatively on the growth of market.
Peripherally Inserted Central Catheters Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1,117.6 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.9% | 2032 Value Projection: | USD 1,782.9 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
AngioDynamics Inc., B. Braun Melsungen AG, Becton, Dickinson and Company, Teleflex Incorporated., Argon Medical Devices, Inc., Cook Medical Inc., ICU Medical, Inc., Medical Components, Inc., Access Vascular, and Vygon (UK) Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Peripherally Inserted Central Catheters Market Segmentation:
Global peripherally inserted central catheters market report is segmented into product type, end user, and region
Based on Product Type, the global peripherally inserted central catheters market is segmented into single lumen, double lumen, and multi Lumen. Among these, double lumen segment is expected to dominate the market over the forecast period due to increasing approval of products in this segment.
Based on Application, the global peripherally inserted central catheters market is segmented into hospitals & clinics, ambulatory surgical centers, and catheterization laboratories. Among these, hospitals and clinics segment is expected to dominate the market over the forecast period due to increasing patient visits in this segment.
Based on Region, the global peripherally inserted central catheters market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa. Among these, North America segment is expected to dominate the market over the forecast period due to increasing launches of products in this region.
Among all segmentation, the product type segment has the highest potential due to increasing approval of products. For instance, on May 05, 2022, Access Vascular, Inc., a U.S.-based medical device company that develops novel bulk hydrophilic-based devices to improve venous access, announced that it has received U.S. Food and Drug Administration 510(k) clearance for its HydroPICC Dual lumen catheter. These devices showed significant reduction in complications such as occlusions, replacements, Deep Vein Thrombosis, and phlebitis. This patented hydrophilic biomaterial catheters mimic the body’s chemistry to dramatically reduce common and costly complications. Given the high utilization and complication rates of standard catheters, the use of Access Vascular, Inc. devices can meaningfully improve patient outcomes. This HydroPICC Dual-Lumen is a 5 French catheter available in multiple kit configurations.
Global Peripherally Inserted Central Catheters Market Cross Sectional Analysis:
Introduction of newer products and technologies by key market players in Europe region is expected to drive growth of product type in the region. For instance, on February 12, 2021, MedLumics, a Spain-based manufacturer of radiofrequency cardiac ablation system’s AblaView, an optically-guided real-time ablation catheter system for the treatment of Atrial Fibrillation (AF), has closed a US$ 19.5 million financing round. Having achieved pre-clinical feasibility in 2020, these proceeds will now enable MedLumics to initiate first in-human regulatory clinical studies and automate scalable product manufacturing.
Global Peripherally Inserted Central Catheters Market: Key Developments
On March 3, 2020, Access Vascular, Inc., a U.S.-based medical device company that develops novel bulk hydrophilic-based devices to improve venous access, announced it has received U.S. FDA clearance for the second generation of its HydroPICC peripherally inserted central catheter (PICC), which has demonstrated an average of 97% less thrombus accumulation on its surface compared to a standard polyurethane catheter. HydroPICC is comprised of Access Vascular’s patented biomaterial platform that combines the superior mechanical properties of polyurethanes with the intrinsically low thrombogenicity of hydrogel. The lubricious and hydrophilic material is designed to repel protein and thrombus development, prevent catheter occlusion, reduce infections, and add flexibility to the catheter.
On April 12, 2023, BACTIGUARD AB, a Sweden-based global med-tech company, announced the launch of a clinical study to compare the Bactiguard’s infection prevention coating technology (BIP CVC) with a non-coated standard catheter. The endpoints of the study will include, catheter-associated blood stream infections and thrombosis rate. The study will be performed at multiple sites in India. The study has been approved by the local regulatory authorities DCGI (Drug Controller General of India). It will be a randomized, controlled multi-center study that will be conducted over one year with the first patient enrolling in the first half of 2023.
On December 5, 2022, Macmillan Cancer Support, a U.K.-based charity and specialist health care provider to people affected by cancer, launched two brand new support videos for people affected by cancer. 'Having your PICC line put in' and 'Having your central line put in' A PICC line is another name for a tube called a catheter. It is implanted in an arm to deliver chemotherapy and other medications. Similarly, a central line is a tube inserted into a chest vein to do the same job. These videos are to spread awareness on when and why this procedure is needed and what to expect. Such informative videos can raise awareness on use of PICC’s.
On January 23, 2023, Inspira-Technologies OXY B.H.N. LTD., Israel based respiratory support Technology Company, announced that the novel convertible dual lumen cannula device and method of use, being developed for the INSPIRA ART System, has been granted a patent by the U.S. Patent and Trademark Office (USPTO). The patent approval includes 20 claims that were found to be novel, with inventive step and industrial applicability.
Global Peripherally Inserted Central Catheters Market: Key Trends
Funding for launching newer devices and techniques
Funding for launching newer devices and techniques can drive growth of market. On February 13, 2020, CloudCath, a U.S.-based maker of a remote monitoring platform for catheter-based treatments, has closed a US$ 12 million Series A financing round. Series A financing round is the first round of venture money a firm raises after seed and angel investors. According to the company, this funding will speed commercialization of the CloudCath system, pending U.S. Food and Drug Administration clearance, and will also support its goal of offering remote monitoring for infectious disease management in multiple additional applications.
Acquisition strategy by key market players
Acquisition strategy by key market players can drive growth of market in forecast period. For instance, On January 6, 2022, Dynamic Infusion Therapy, LLC, a U.S. based on-demand outsourced provider of vascular access insertion services, announced the acquisition of Lifeline PICC, a U.S.-based provider of vascular access services focused at healthcare organizations. In connection with the transaction, Dynamic Infusion Therapy, LLC will welcome the experienced Lifeline PICC nurses to its team and will be providing services to several prominent healthcare clients in the Tulsa area. Both companies will share a clinical model that focuses on lowering customer costs while providing better patient outcomes.
Global Peripherally Inserted Central Catheters Market: Restraints
Recalls of products
The recalls of peripherally inserted central catheters is expected to hamper the global peripherally inserted central catheters market growth. For instance, on December 16, 2022, a U.S. FDA announced Teleflex Incorporated, a U.S.-based global provider of medical technologies and Arrow International, Inc., a U.S.-based medical equipment & devices manufacturer, recalling both the Arrow MAC Two-Lumen Central Venous Access Kit and the Arrow Pressure Injectable Arrowg+ard Blue Plus Three-Lumen Central Venous Catheter Kit. The reason for the recall is the risk of a cross-lumen leak caused by inadequate connections between the top and bottom housings of the Micro Clave Clear Connectors included in the kits. Device may reportedly cause bleeding, fluid leakage, delayed treatment, infection, air embolism, death or other serious injuries in patients treated with these device, however, no injuries or deaths have been reported.
To counterbalance this restraint, more research & development activities should be carried out to avoid recalls.
Lack of awareness of central venous catheters
In emerging economies, lack of awareness of central venous catheters is expected to hamper the global peripherally inserted central catheters market growth. For instance, according to an article published in the Journal of Hospital Medicine on February 25, 2020, 375 patient records were accessed and the providers interviewed regarding awareness of presence of a PICC in its patients. Results demonstrated that many physicians are not aware of whether the patient has a central line. Such unawareness may lead to unnecessary complications which can restrain growth of market.
To counterbalance this restrain, more training to caregiver and physicians should be given.
Global Peripherally Inserted Central Catheters Market- Key Players
Major players operating in the global peripherally inserted central catheters market include AngioDynamics Inc., B. Braun Melsungen AG, Becton, Dickinson and Company, Teleflex Incorporated., Argon Medical Devices, Inc., Cook Medical Inc., ICU Medical, Inc., Medical Components, Inc., Access Vascular, and Vygon (UK) Ltd.
*Definition: A peripherally inserted central catheter (PICC), also called a PICC line, is a long, thin tube that's inserted through a vein in the arm and passed through to the larger veins near the heart. A peripherally inserted central catheter (PICC) is used as a medium to insert medications or liquid nutrition. A PICC line can help to avoid the pain of frequent needle sticks and reduce the risk of irritation to the smaller veins in the arms. A PICC line is usually intended to be temporary and might be an option if treatment is expected to last up to several weeks. A PICC line requires careful care and monitoring for complications, including infection and blood clots. A peripherally inserted central catheter (PICC) line is recommended for cancer treatment, liquid nutrition, infection treatment, and other medications.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients