Perfusion System Market Size and Forecast – 2025 – 2032
The Global Perfusion System Market size is estimated to be valued at USD 1.6 billion in 2025 and is expected to reach USD 3.2 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 10.4% from 2025 to 2032.
Global Perfusion System Market Overview
Perfusion systems are advanced medical devices designed to circulate blood or other fluids through organs or tissues during surgical procedures. These systems include pumps, oxygenators, heat exchangers, and monitoring components that maintain controlled flow, temperature, and oxygenation. Perfusion products are engineered for precision and safety, enabling procedures such as cardiopulmonary bypass, organ preservation, and extracorporeal life support. Modern systems incorporate real-time sensors, digital monitoring, and user-friendly interfaces to optimize patient outcomes and minimize procedural risks.
Key Takeaways
The roller pump segment commands the highest market share, driven by its reliability and extensive use in cardiac surgeries, presenting continued growth potential.
Hospitals remain the most significant end users, accounting for a majority of the market revenue, backed by rising investments in surgical infrastructure.
North America holds a dominant industry share in the market, credited to its advanced healthcare delivery system and strong regulatory environment for medical devices.
Asia Pacific stands as the fastest-growing region, exhibiting a CAGR surpassing 11%, fueled by expanding healthcare access and government initiatives promoting cardiovascular health.
Perfusion System Market Segmentation Analysis
To learn more about this report, Download Free Sample
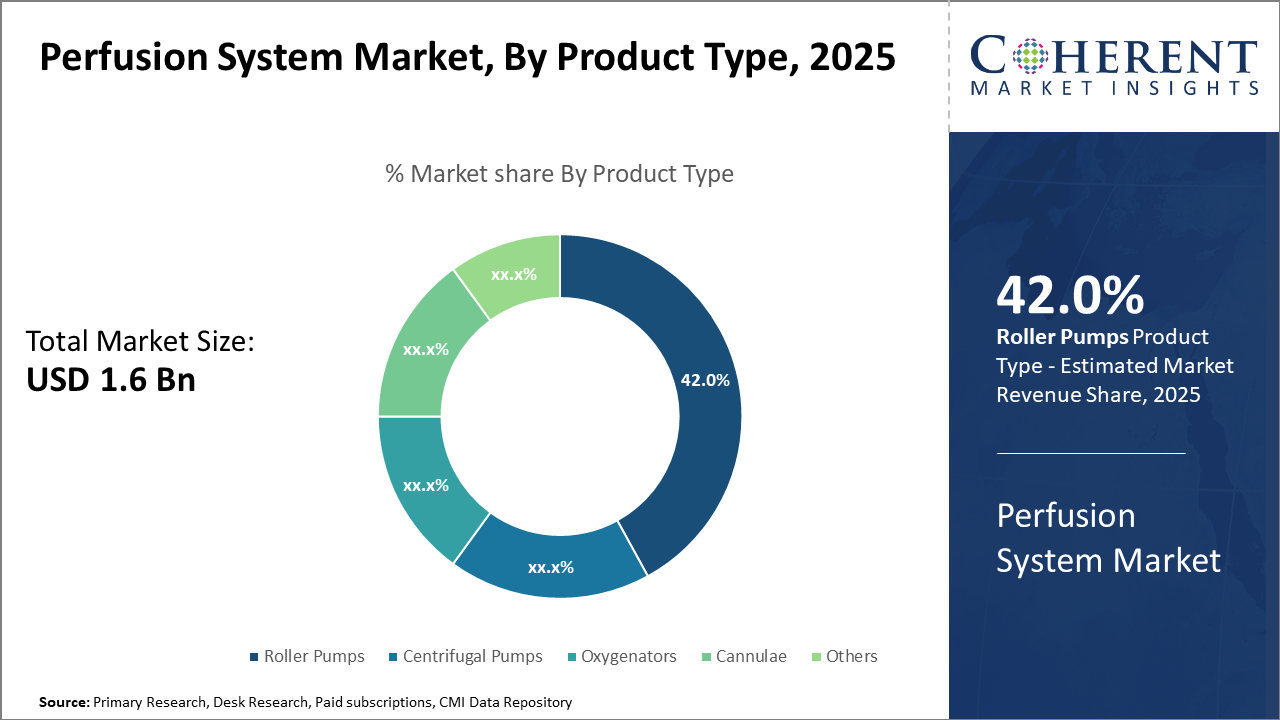
Perfusion System Market Insights, By Product Type
Roller Pumps dominate the market share with 42% due to their robust performance in diverse surgical applications, ease of use, and reliability, sustaining their dominant position across healthcare facilities globally. Centrifugal pumps represent the fastest-growing subsegment, driven by technological innovations that provide improved shear stress reduction and enhanced patient safety during surgeries. Oxygenators and Cannulae continue to hold substantial sub-market presence, serving critical functions in oxygen exchange and blood flow management.
Perfusion System Market Insights, By Application
Cardiac Surgery dominates the market share at approximately 60%. This dominance is due to the high volume of cardiovascular procedures requiring complex perfusion techniques and continuous technological innovations that improve clinical outcomes. Organ Preservation represents the fastest-growing segment, propelled by the increasing global transplant surgeries and advances in machine perfusion technologies, enhancing graft survival rates.
Perfusion System Market Insights, By End User
Hospitals dominate the market share due to their substantial surgical volumes and well-established operational frameworks capable of integrating cutting-edge perfusion systems. Hospitals invest heavily in renewing cardiac surgery equipment, maintaining their dominant share. Ambulatory Surgical Centers and Specialty Clinics are the fastest-growing subsegments as they increasingly adopt minimally invasive and outpatient procedures requiring portable and cost-effective perfusion devices.
Perfusion System Market Trends
The market trend indicates a growing inclination toward automation and digital integration to optimize surgical outcomes.
In 2024, AI-based perfusion monitoring systems demonstrated improved precision, which was rapidly adopted by leading cardiac centers in North America.
Disposable perfusion components became essential following the pandemic, heightening demand worldwide.
Additionally, the shift toward portable perfusion technologies aligns with the growing prevalence of minimally invasive surgeries in developing economies such as India and Brazil, reflecting a paradigm shift in surgical care delivery.
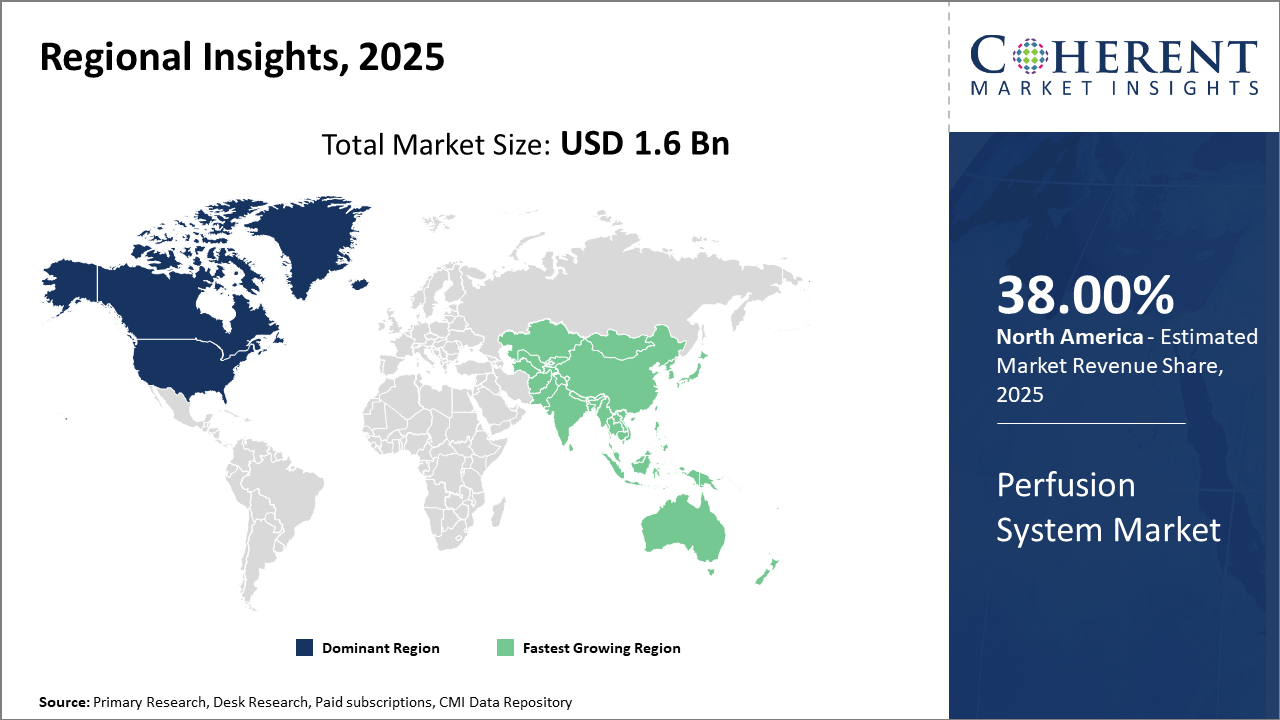
Perfusion System Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Perfusion System Market Analysis and Trends
In North America, the dominance in the Perfusion System market is underpinned by an extensive healthcare ecosystem, well-established regulatory frameworks, and the presence of key manufacturers. The region accounts for nearly 38% of the global market share, driven by high procedure volumes and healthcare expenditure. Innovations and early adoption of advanced perfusion devices by leading hospitals further reinforce the region’s commanding position.
Asia Pacific Perfusion System Market Analysis and Trends
Meanwhile, the Asia Pacific region exhibits the fastest growth with a CAGR exceeding 11%, fueled by increasing cardiac surgery volumes and government-supported healthcare reforms across China and India. Rising awareness and expanding private healthcare infrastructure are leading to heightened investments in perfusion systems, amplifying market revenue growth.
Perfusion System Market Outlook for Key Countries
USA Perfusion System Market Analysis and Trends
The U.S. perfusion system market remains a global frontrunner, bolstered by significant healthcare infrastructure investments and technological innovation. Regulatory support from bodies like the FDA expedited the approval of AI-integrated perfusion pumps in 2024. Major players such as Medtronic and LivaNova have launched advanced centrifugal pump devices, contributing to a year-over-year revenue increase exceeding 9%. The prevalence of cardiac diseases and large patient pools for organ transplants further sustain this growth trajectory.
India Perfusion System Market Analysis and Trends
India's market is rapidly evolving, driven by increasing cardiac healthcare expenditures and burgeoning private hospital networks. In 2025, government programs focusing on non-communicable diseases led to a 12% rise in cardiac surgical procedures, significantly elevating demand for advanced perfusion systems. Local manufacturing capabilities are expanding, coupled with imports of sophisticated devices from established global manufacturers, resulting in a competitive market environment and increasing market share.
Analyst Opinion
Increasing adoption of minimally invasive surgeries globally is a strong demand-side indicator accelerating the market growth. The rising number of cardiac surgeries—such as coronary artery bypass grafting and heart valve surgeries—in the U.S. alone increased by approximately 8% in 2024 compared to the previous year, directly increasing the demand for advanced perfusion systems. Moreover, expanding surgical volumes in Asia Pacific reflect a similar upward momentum, contributing significantly to industry size expansion.
Supply-side capacities have improved notably with the introduction of modular and portable perfusion systems, resulting in a 15% rise in production capacity among top manufacturing hubs in 2025. Pricing dynamics remain competitive due to technological advancements and increased automation in assembly lines, reducing manufacturing costs by up to 12%, thereby enhancing market revenue for key players.
The cardiac care segment continues to dominate end-user application sectors with a market share of around 60% in 2025. Hospitals are increasingly investing in upgraded perfusion devices to improve patient outcomes, as reflected by a 20% increase in capital expenditure on perfusion technologies reported by healthcare institutions across Europe and North America in 2024.
Export trends indicate strong intercultural collaboration with emerging economies such as India and Brazil stepping up procurement of high-end perfusion systems, boosting exports by nearly 10% in 2025. This reflects global market growth strategies focusing on cross-border partnerships and expanding market scope through local distributor channels.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 1.6 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 10.4% | 2032 Value Projection: | USD 3.2 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Terumo Corporation, Medtronic plc, LivaNova PLC, Getinge AB, Sorin Group (now part of LivaNova), Maquet Getinge Group, Nipro Corporation, Edwards Lifesciences Corporation, Lippincott Perfusion Supplies, Medos Medizintechnik AG, XX Medical Systems. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Perfusion System Market Growth Factors
Technological Innovations: The introduction of portable and user-friendly perfusion devices with enhanced safety features is revolutionizing surgical procedures, making perfusion systems more accessible in remote and ambulatory settings. For instance, handheld perfusion pumps gained a 15% adoption rate in new hospitals during 2024.
Rising Organ Transplantations: Increasing organ transplant rates globally, driven by improved healthcare infrastructure and awareness, are fuelling demand for advanced organ preservation perfusion systems. Brazil’s kidney transplantation numbers rose by 9% in 2024, correlating with increased procurement of associated perfusion technologies.
Government Support and Healthcare Spending: Expanding government funding and initiatives targeting cardiovascular disease diagnostics and treatment, such as the U.S. Heart and Stroke Prevention Act enhancements in 2025, have provided robust market growth impetus, supporting enhanced adoption of perfusion systems across healthcare facilities.
Surge in Cardiac Surgeries: The global rise in cardiac surgeries has driven hospitals to invest in upgraded perfusion technologies. Data from the European Society of Cardiology in 2025 notes a 7% increase in cardiac interventions, directly boosting equipment demand.
Perfusion System Market Development
In August 2025, LivaNova announced the commercial launch of its Essenz™ Perfusion System in China, marking a major expansion following its successful European debut in 2023. The Essenz System integrates advanced patient monitoring, real-time data analytics, and customizable workflow technology to support optimized perfusion management during cardiopulmonary bypass procedures. Equipped with a next-generation heart-lung machine and the Essenz Perfusion Data Management System, it enables clinicians to make informed, data-driven decisions to improve patient outcomes.
In January 2025, Paragonix Technologies announced the first-in-human use of its KidneyLogic™ Renal Preservation System, a novel hypothermic perfusion platform designed to enhance the preservation and transport of donor kidneys for transplantation. The system maintains optimal perfusion parameters, temperature control, and real-time organ monitoring to minimize ischemic injury and improve post-transplant graft function. This milestone marks a significant step in expanding Paragonix’s portfolio beyond heart and lung preservation, where its SherpaPak® systems are already widely adopted.
Key Players
Leading companies of the market include:
Terumo Corporation
Medtronic plc
Getinge AB
Sorin Group (now part of LivaNova)
Maquet Getinge Group
Nipro Corporation
Edwards Lifesciences Corporation
Lippincott Perfusion Supplies
Medos Medizintechnik AG
XX Medical Systems
Several leading companies have aggressively adopted acquisition and technological innovation strategies. For example, LivaNova PLC’s acquisition of Sorin Group allowed it to expand its perfusion system portfolio, driving revenue growth by 18% in 2024. Meanwhile, Terumo Corporation invested heavily in R&D for centrifugal pump systems, launching a new product line that reduced complications during cardiac surgery, which led to a 12% increase in global market share in 2025.
Perfusion System Market Future Outlook
In the future, the market is expected to continue expanding with the introduction of smart perfusion systems featuring AI-assisted monitoring, predictive analytics, and enhanced user interfaces. Demand will be fueled by rising cardiovascular disease prevalence, growing organ transplantation procedures, and an increasing number of minimally invasive surgeries requiring precise perfusion support. Emerging markets are likely to experience accelerated growth due to healthcare modernization and increased investment in surgical infrastructure. Integration with telemedicine and remote monitoring solutions will further enhance procedural efficiency and patient safety, reinforcing market growth over the next decade.
Perfusion System Market Historical Analysis
The perfusion system market has evolved significantly over the past few decades, driven by the growing complexity of cardiovascular and organ transplant procedures. Initially, perfusion systems were largely manual, limited to basic cardiopulmonary bypass functions, and confined to specialized hospitals. Advancements in automation, integrated monitoring, and real-time control enhanced safety, efficiency, and patient outcomes, leading to broader adoption in surgical centers globally. Increasing awareness of organ preservation techniques and extracorporeal life support systems also contributed to the market’s steady growth. Regional healthcare infrastructure improvements and the establishment of dedicated cardiac centers accelerated the integration of advanced perfusion technologies into mainstream clinical practice.
Sources
Primary Research interviews:
Cardiac Surgeons
Perfusionists
Transplant Surgeons
Biomedical Engineers
Hospital Procurement Managers
Databases:
PubMed
FDA Medical Device Database
ECRI Institute Reports
Magazines:
Medical Device Network
Cardiology Today
Perfusion & Technology Today
Journals:
Journal of ExtraCorporeal Technology
The Journal of Thoracic and Cardiovascular Surgery
Annals of Thoracic Surgery
Newspapers:
The New York Times (Health)
The Guardian (Science)
The Washington Post (Health
The Wall Street Journal (Healthcare)
Associations:
American Society of ExtraCorporeal Technology (AmSECT)
Society of Thoracic Surgeons (STS)
International Society for Heart and Lung Transplantation (ISHLT)
American Heart Association (AHA)
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients