Percutaneous transluminal angioplasty balloons catheter market is estimated to be valued at USD 1.99 Bn in 2025 and is expected to reach USD 3.71 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 9.3% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
Percutaneous transluminal angioplasty balloons catheter industry can witness robust growth over the forecast period due to technological advancements such as drug-coated balloons and balloons with higher flexibility. Rising prevalence of cardiovascular diseases along with growing preference for minimally invasive procedures can drive the market growth. Furthermore, increasing healthcare spending especially in emerging economies and rising geriatric population can also drive the market growth in the near future. However, high costs associated with these devices can hamper its widespread adoption.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
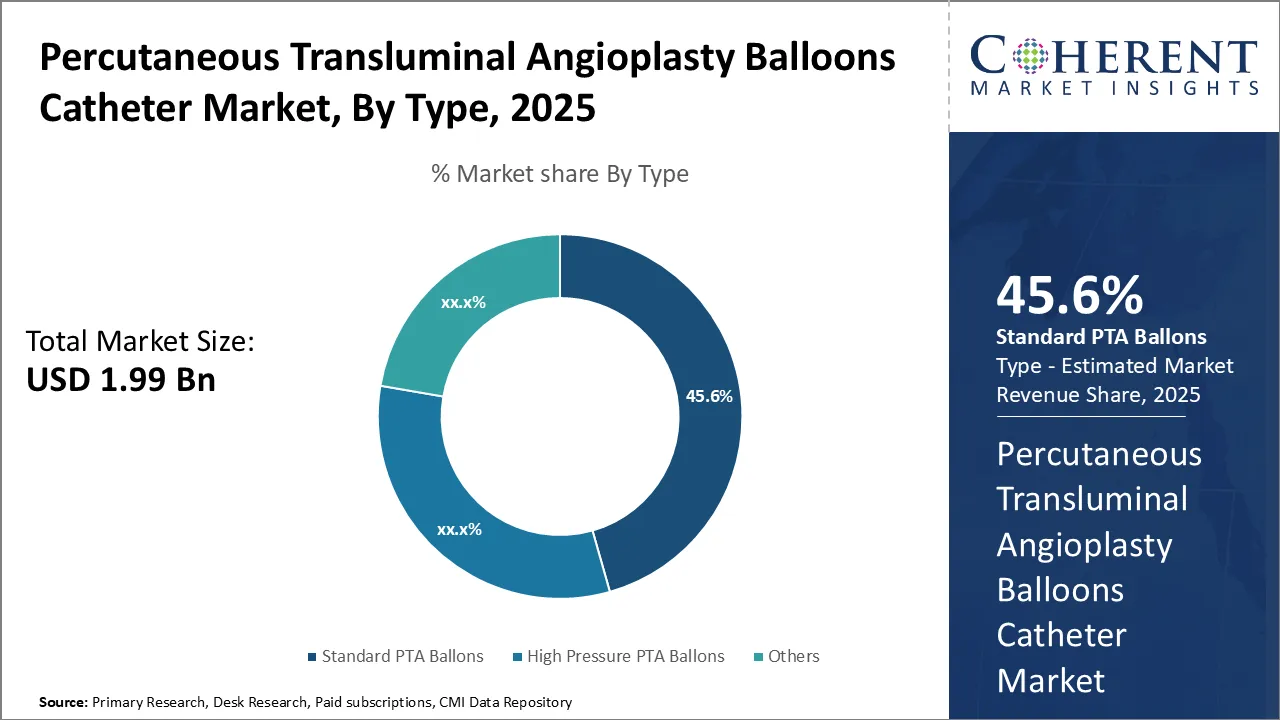
Insights, By Type- Affordability and Extensive Usage Drives Standard PTA Ballons Segment
In terms of type, standard PTA ballons segment is estimated to contribute the highest market share of 45.6% in 2025, due to its affordability and extensive usage. Preferred by both physicians and patients, these balloons have a long-standing history of effective use in angioplasty procedures. Their cost-effectiveness, proven safety, and availability in various sizes makes them suitable for diverse artery anatomies, ensuring reliable outcomes for peripheral vascular and coronary conditions.
Insights, By Material Type- Polyurethane Emerges as Material of Choice Due to Superior Properties
In terms of material type, polyurethane segment is estimated to contribute the highest market share of 62.1% in 2025, owing to its superior material properties. Its thin yet durable construction allows for effective tracking through tortuous vessels without damage. Polyurethane balloons can inflate at high pressures, facilitating artery widening, and their biocompatibility minimizes inflammation risk. With smooth surface, these balloons enable minimally traumatic interventions, making polyurethane the preferred choice over nylon and other polymers in angioplasty procedures.
Insights, By Application- Peripheral Artery Disease Dominates Application Segment Due to High Prevalence
In terms of application, peripheral artery disease segment is estimated to contribute the highest market share of 69.8% in 2025, due to its high global prevalence. This condition arises from plaque accumulation in arteries supplying blood to the hips and legs, with risk factors including age, diabetes, and smoking. The demand for specialized below-the-knee artery clearing has boosted need for targeted low-profile PTA balloons and catheters, solidifying PAD as the primary application for percutaneous angioplasty.
Need a Different Region or Segment? Download Free Sample
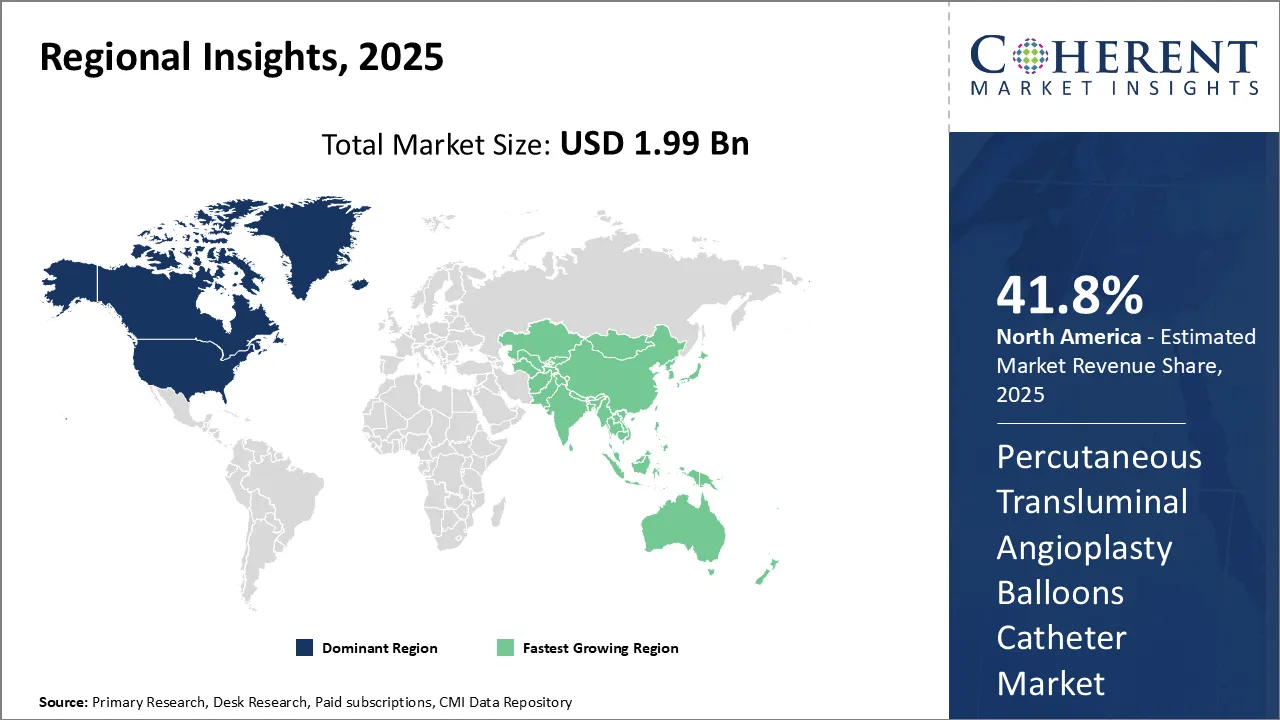
Dominating Region- North America
North America is expected to dominate the percutaneous transluminal angioplasty balloons catheter industry, with an estimated market share of 41.8% in 2025, due to factors such as presence of well-established healthcare infrastructure, advanced medical technologies, and high healthcare expenditure. Major players in the region such as Medtronic and Boston Scientific Corporation have been introducing innovative products, thus, driving market growth.
Fastest-Growing Region- Asia Pacific
Asia Pacific region exhibits the fastest growth in percutaneous transluminal angioplasty balloons catheter industry, with an estimated market share of 19.9% in 2025. This can be attributed to increasing patient awareness, improving access to healthcare, and supportive government policies promoting local manufacturing. Leading global companies are focusing on their expansion strategies in the region to tap into high-potential market.
Percutaneous Transluminal Angioplasty Balloons Catheter Market Outlook for Key Countries
Products innovation in the U.S.
The U.S. market for percutaneous transluminal angioplasty balloons catheters is driven by top firms that are heavily investing in research and development to develop advanced solutions. This focus on innovation drives growth and enhances treatment options in the healthcare sector.
Growing geriatric population in China
China market for percutaneous transluminal angioplasty balloons catheters is experiencing rapid growth due to growing geriatric population, increased healthcare expenditure, and domestic manufacturers providing cost-effective products. This combination enhances access to vital medical interventions, catering to rising demand for effective cardiovascular treatments among the elderly.
Technological advancements in Japan
Japan percutaneous transluminal angioplasty balloons catheter market growth is driven by technologically advanced healthcare system and a universal health insurance framework. This system ensures equitable access to high-quality medical care, facilitating the adoption of innovative medical technologies and improving patient outcomes across the nation.
Widening patient pool and collaborative efforts in India
India's market for percutaneous transluminal angioplasty balloons catheters is experiencing significant growth due to widening patient pool and collaborative efforts from both the public and private sectors to improve accessibility. These initiatives aim to enhance healthcare infrastructure and ensure that advanced medical treatments are available to a broader population.
Get actionable strategies to beat competition: Download Free Sample
Top Strategies Followed by Percutaneous Transluminal Angioplasty Balloons Catheter Market Players
Emerging Startups in the Percutaneous Transluminal Angioplasty Balloons Catheter Market
Key Takeaways from Analyst
Percutaneous Transluminal Angioplasty Balloons Catheter Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1.99 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.3% | 2032 Value Projection: | USD 3.71 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
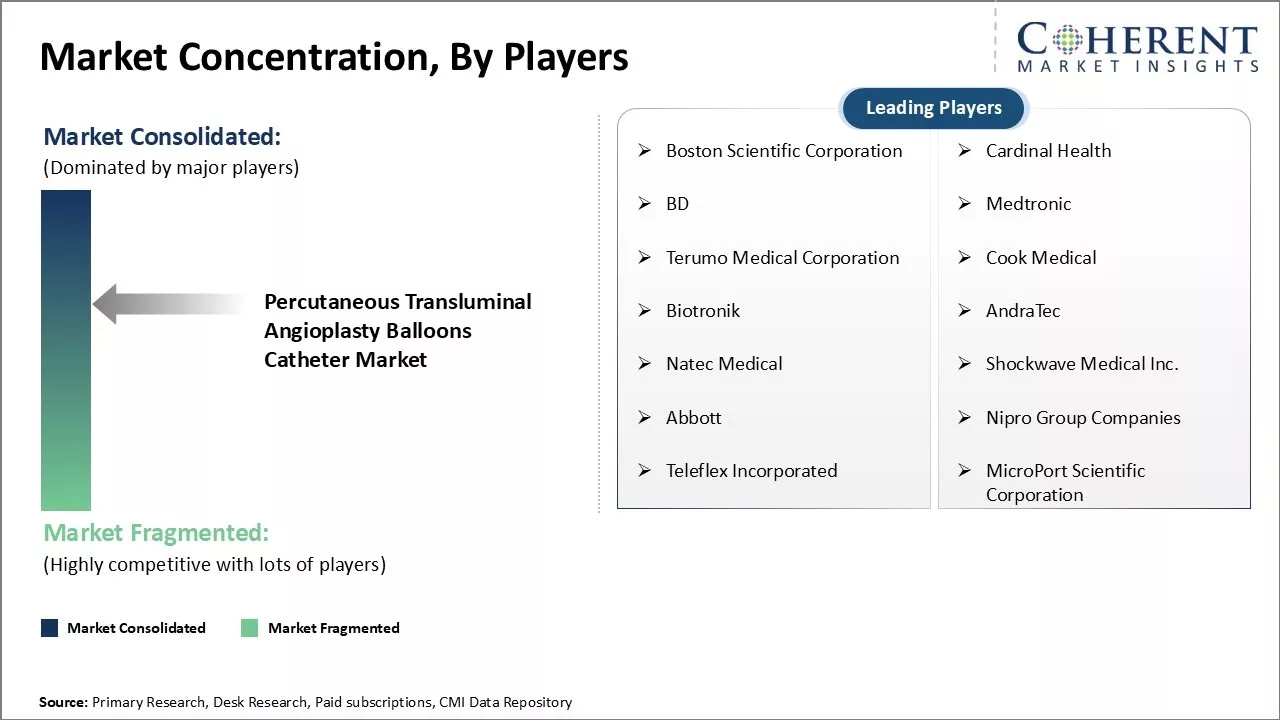
Boston Scientific Corporation, Cardinal Health, BD, Medtronic, Terumo Medical Corporation, Cook Medical, Biotronik, AndraTec, Natec Medical, Shockwave Medical Inc., Abbott, Nipro Group Companies, Teleflex Incorporated, MicroPort Scientific Corporation |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
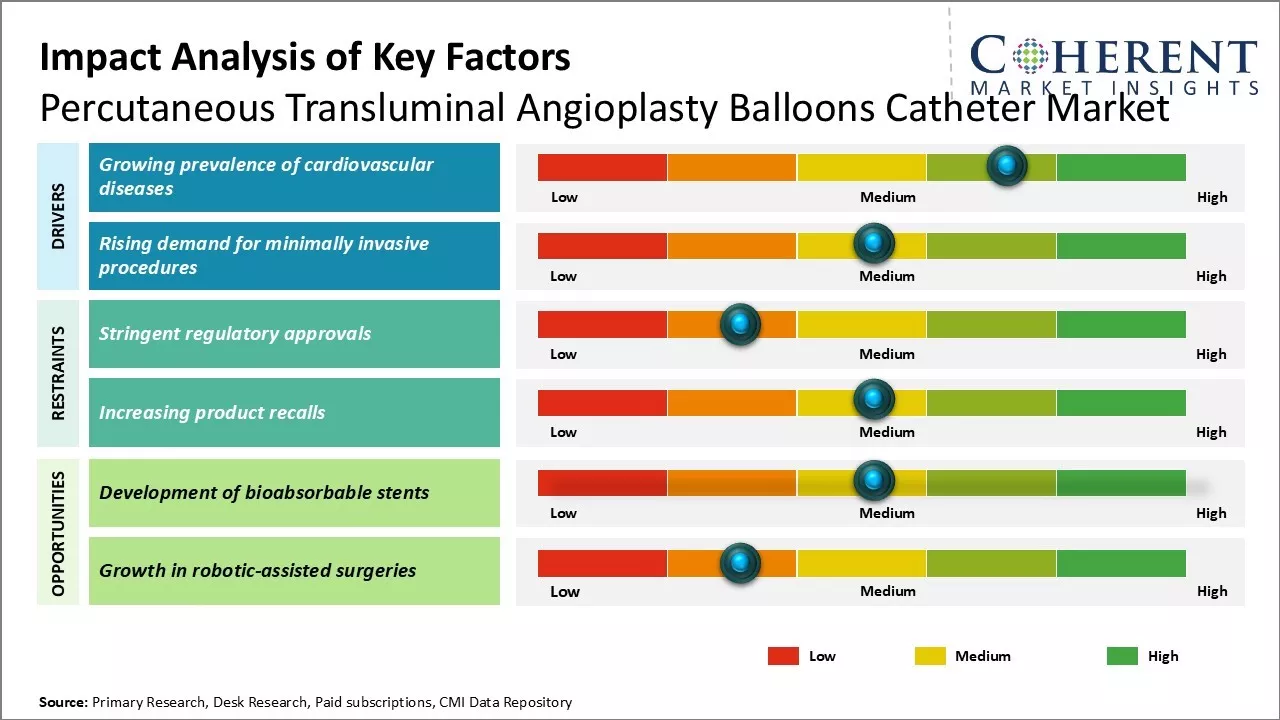
Market Driver- Rising demand for minimally invasive procedures
The shift towards minimally invasive surgical approaches, including angioplasty, is transforming medical practice. This method reduces recovery time, trauma, and complications for patients while lowering healthcare costs. Angioplasty is increasingly preferred over traditional bypass surgeries due to less scarring, reduced bleeding, and shorter hospital stays. As patient awareness grows and device manufacturers promote these benefits, there will be demand for angioplasty, driving innovation in catheter technology and improving procedural success rates across diverse vascular cases.
Market Challenge- Stringent regulatory approvals
Percutaneous transluminal angioplasty balloons catheter market faces challenges like stringent regulatory approval process for new products. Regulatory bodies like the U.S. FDA and European Medicines Agency require extensive clinical trials to demonstrate safety and efficacy, leading to delays in market entry. Any modifications to approved products may necessitate further approvals, increasing costs and timelines. The varying compliance requirements across countries complicate product development and hinder broader market penetration of innovative technologies.
Market Opportunity- Development of bioabsorbable stents
Development of bioabsorbable stents offers significant opportunities for the percutaneous transluminal angioplasty balloons catheter market. These stents dissolve over time, reducing the need for lifelong dual antiplatelet therapy and minimizing risks like late stent thrombosis. With promising clinical outcomes and ongoing R&D efforts, major companies are focused on advancing this technology, which could attract further investment and drive growth in related products, including balloon catheters.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients