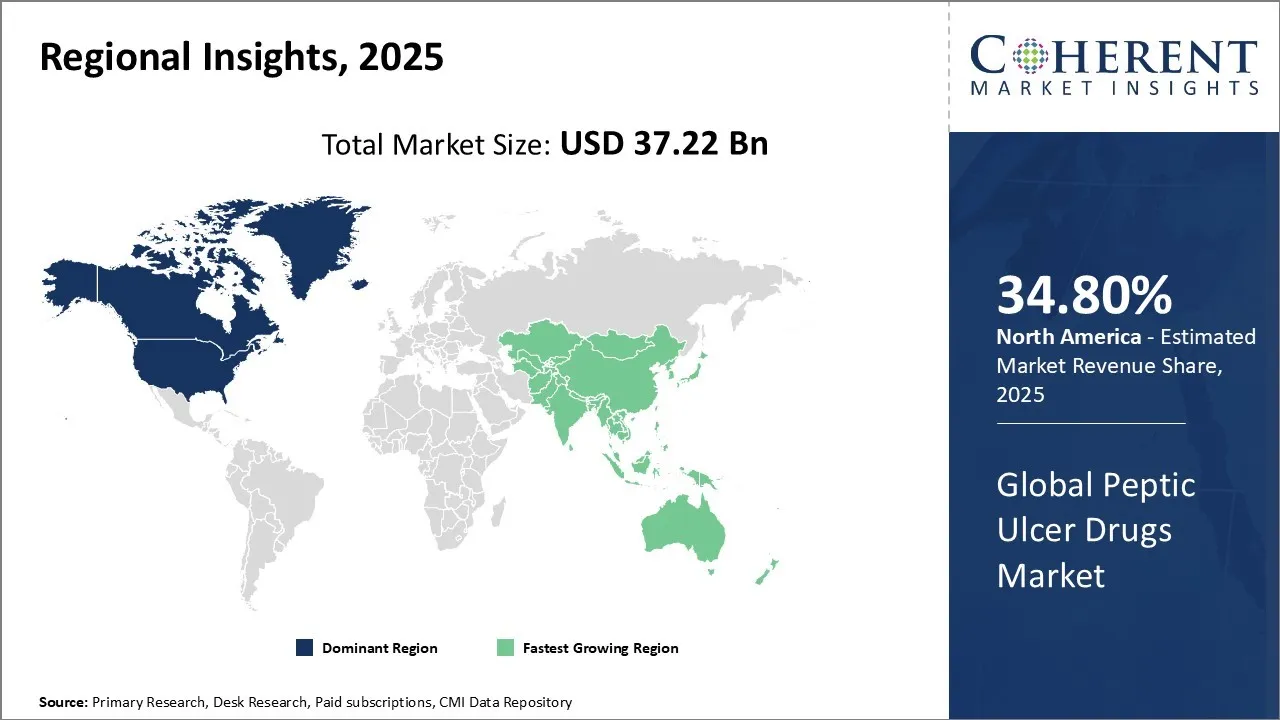
Peptic Ulcer Drugs Market is estimated to be valued at USD 37.22 Bn in 2025 and is expected to reach USD 49.31 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 4.1% from 2025 to 2032.
The growth of the Peptic Ulcer Drugs Market’s growth is fueled by the increasing prevalence of Helicobacter pylori infections, widespread NSAID usage, and aging population shifts towards medical therapies.
|
Current Events |
Description and its impact |
|
Technological Advances and Pharmaceutical Innovations |
|
|
Shifts in Patient Preferences and Physician Treatment Patterns |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Based on Product Type, the market is segmented into steroids, proton pump inhibitors, potassium-competitive acid blockers (p-cab), antacids, h2- antagonists, antibiotics, and ulcer protection. Out of which, the Proton Pump Inhibitors segment is expected to dominate the global peptic ulcer drugs market during the forecast period and this is attributed to an increase in the product launches by key market players. In June 2025, Eisai Co., Ltd. launched “Pariet® S,” the first proton pump inhibitor (PPI) approved as an over-the-counter (OTC) medicine in Japan, effective in relieving severe heartburn and stomach pain from acid reflux. It is now available at pharmacies and drugstores nationwide. This is further propelling the peptic ulcer drugs market share.
Based on Disease Indication, the market is segmented into gastritis, gastric ulcer, duodenal ulcer, and gastroesophageal reflux disease (GERD). Out of which, the duodenal ulcer segment is expected to dominate the global peptic ulcer drugs market during the forecast period and this is attributed to an increase in the global prevalence of gastric ulcer. For instance, Akums launched Combikit for Duodenal Ulcer where the Combikit is approved by the CDSCO for healing duodenal ulcers associated with Helicobacter pylori in patients with active or healed peptic ulcer.
Based on the Distribution Channel, the market is segmented into Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies. Out of which, the hospital pharmacies segment is expected to dominate the market over the forecast period and this is attributed to an increase in the number of hospital pharmacies. For instance, Apollo HealthCo Ltd is expanding its pharmacy network in West Bengal, aiming to establish numerous new outlets across the state. Such initiatives are adding to the peptic ulcer drugs market demand. Apart from this, there is a growing penetration towards e-pharmacy, which is further acting as a major growth-inducing factor.
To learn more about this report, Download Free Sample
Among all regions, North America is expected to dominate the global market with a share of 34.80% over the forecast period. This is attributed to North America holding a large market share and an increase in product approval by the regulatory bodies in this region. For instance, in June 2025, Zydus Lifesciences, a pharmaceutical company, announced that the company had received approval from the U.S. Food and Drug Administration (FDA) to market Famotidine tablets in the strengths of 20 mg and 40 mg. Famotidine is a histamine H2 receptor blocker. It works by reducing the amount of acid in the stomach.
The Europe peptic ulcer drugs market is growing steadily, driven by an increasing prevalence of peptic ulcers caused by NSAID use, and lifestyle factors. Advancements in drug formulations, especially proton pump inhibitors (PPIs) and H2 receptor antagonists, improve treatment efficacy and patient adherence. The rise of over-the-counter (OTC) availability for some ulcer medications also enhances accessibility, boosting market demand. Ranbaxy Laboratories announced the launch of generic esomeprazole tablets in 20 mg and 40 mg strengths for ulcer treatment in the United Kingdom.
The United States Peptic Ulcer Drug Market is seeing significant growth due to the increasing prevalence of H.pylori infections and widespread NSAID/aspirin use especially among older adults and the fueling demand, as these are the leading causes of ulcers in the U.S. population. For instance, Phathom Pharmaceuticals announced FDA Approval of Reformulated Vonoprazan Tablets for VOQUEZNA® TRIPLE PAK® (vonoprazan, amoxicillin, clarithromycin) and VOQUEZNA® DUAL PAK® for the Treatment of H. pylori Infection in Adults.
The rise in the peptic ulcer drugs market in India is driven by the increasing prevalence of Helicobacter pylori infections and growing gastrointestinal complications due to changing dietary habits and stress. Expanding access to healthcare in rural areas and rising awareness about gastrointestinal disorders are further propelling demand. For, instance Dr. Reddy’s, in innovation with Takeda, launched Vonoprazan (VONO) in India, a potassium-competitive acid blocker (PCAB) for treating acid peptic disorders like reflux esophagitis.
The increasing prevalence of peptic ulcers is a major factor boosting the growth of the global peptic ulcer drugs market over the forecast period. For instance, data published in April 2021, stated that in the U.S., peptic ulcer disease affects approximately 4.6 million people every year.
An increase in the research and development activities by the key players in the market is expected to drive the growth of the market over the forecast period. For instance, on January 4, 2023, Tanta University, based in Egypt, initiated a clinical study titled ‘Diosmin as Adjuvant Therapy in Treatment of Non-bleeding Peptic Ulcer.’ The study is expected to be completed by June 2025.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 37.22 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 4.1% | 2032 Value Projection: | USD 49.31 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Daewoong Pharmaceutical Co., Ltd, Takeda Pharmaceutical Co., Ltd., Pfizer Inc., AstraZeneca plc., RedHill Biopharma, Cadila Healthcare Ltd., Novitium Pharma LLC., Boehringer Ingelheim GmbH, Eisai Co. Ltd., Yuhan Corporation, GlaxoSmithKline plc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
In the product type segment, the proton pump inhibitors segment held a dominant position in the Asia Pacific region due to the increasing adoption of inorganic growth strategy agreements by key market players. For instance, in March 2021, Daiichi Sankyo Co., Ltd., a pharmaceutical company, made an agreement to transfer the marketing and distribution rights for the proton pump inhibitor Nexium capsules 10 mg and 20 mg and Nexium granules for suspension 10 mg and 20 mg (esomeprazole magnesium) to AstraZeneca in Japan.
In the disease indication segment, the Gastric Ulcer segment held a dominant position in the North American region due to the increase in research and development activities by key market players. For instance, on June 9, 2020, Spital Limmattal Schlieren, a general and teaching hospital based in Switzerland, initiated a clinical study titled ‘Functional Changes in the Stomach and Esophagus After One Anastomosis Gastric Bypass- OAGB- BiFlux Trial.’ The study is estimated to be completed on May 28, 2028.
In April 2025, Sun Pharmaceutical Industries launched FEXUCLUE, a 40 mg potassium-competitive acid blocker (P-CAB), in India. Approved for treating adults with erosive esophagitis of all grades, FEXUCLUE contains Fexuprazan and targets acid-related disorders such as GERD and erosive esophagitis. It is also under investigation for preventing NSAID-induced peptic ulcers and gastritis.
In September 2024, Jeil Pharmaceutical unveiled Jaqbo, a new potassium-competitive acid blocker to enter peptic ulcer drug market.
In June 2024, Mankind Pharma & Takeda signed a non-executive patent license for vonoprazan in India. This enables Mankind to commercialize Takeda’s first-in-class P-CAB (potassium-competitive acid blocker) for duodenal and peptic ulcers.
In September 2024, Hk inno. N gained approvals in six Latin American countries for K-CAB, a new P-CAB targeting GERD and peptic ulcers for a new drug for gastroesophageal.
In September 2024, Jeil Pharmaceuticals launched Jaqbo(zastaprazan citrate) in South Korea, another P-CAB entering the gastric/peptic ulcer segment.
The major factors that can hamper the growth of the global peptic ulcer drugs market over the forecast period include side effects associated with the drugs used to treat peptic ulcers. Prolonged use of peptic ulcer drugs such as proton pump inhibitors and antibiotics may lead to an increased risk of hip bone fracture as the ability to absorb calcium and iron decreases. Other side effects include the risk of opportunistic infections with S. pneumonia and C. difficult, owing to diminished natural gut flora. Such a scenario is expected to hamper the growth of the global peptic ulcer drugs market.
*Definition: Peptic ulcers are open sores that develop on the inside lining of your stomach and the upper portion of your small intestine. The most common symptom of a peptic ulcer is stomach pain. Peptic ulcers include: Gastric ulcers that occur on the inside of the stomach.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients