Pemphigus Vulgaris Treatment Market Size and Forecast – 2025 – 2032
The Global Pemphigus Vulgaris Treatment Market size is estimated to be valued at USD 320 million in 2025 and is expected to reach USD 630 million by 2032, exhibiting a compound annual growth rate (CAGR) of 10.2% from 2025 to 2032.
Global Pemphigus Vulgaris Treatment Market Overview
Treatment products for pemphigus vulgaris primarily target immune modulation to prevent autoantibody-mediated skin blistering. Historically, corticosteroids and immunosuppressants such as azathioprine were the mainstay therapies, providing symptomatic relief but with long-term side effects. In recent years, biologics like rituximab have revolutionized treatment by selectively targeting B-cells, achieving faster remission and fewer relapses. Adjunct therapies, including intravenous immunoglobulin (IVIG) and plasma exchange, are also used in refractory cases. Ongoing innovation focuses on monoclonal antibodies, targeted immunotherapies, and biosimilar development to enhance efficacy and reduce treatment costs, marking a significant step toward precision dermatological therapeutics.
Key Takeaways
The Biologics segment maintains dominance, reinforced by innovative therapeutic agents capturing over 50% industry share, driven by robust clinical efficacy data.
Hospitals remain the largest end-users due to their established infrastructure and the complexity of managing Pemphigus Vulgaris cases, accounting for 60% market share.
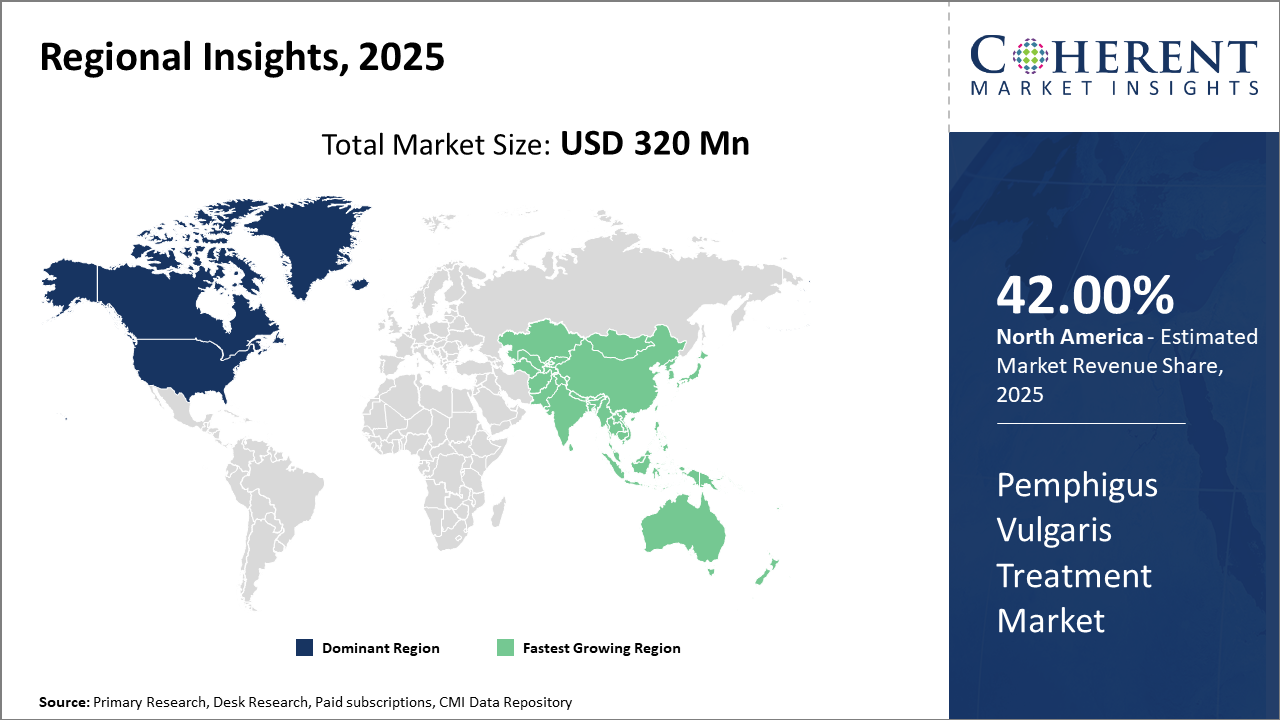
North America retains its market share leadership, owning approximately 42% of global revenue, fueled by advanced healthcare systems and strong reimbursement frameworks.
Asia Pacific is the fastest-growing region, with a CAGR exceeding 12%, propelled by expanding healthcare access, improved disease awareness, and favorable government initiatives in countries like China and India.
Pemphigus Vulgaris Treatment Market Segmentation Analysis
To learn more about this report, Download Free Sample
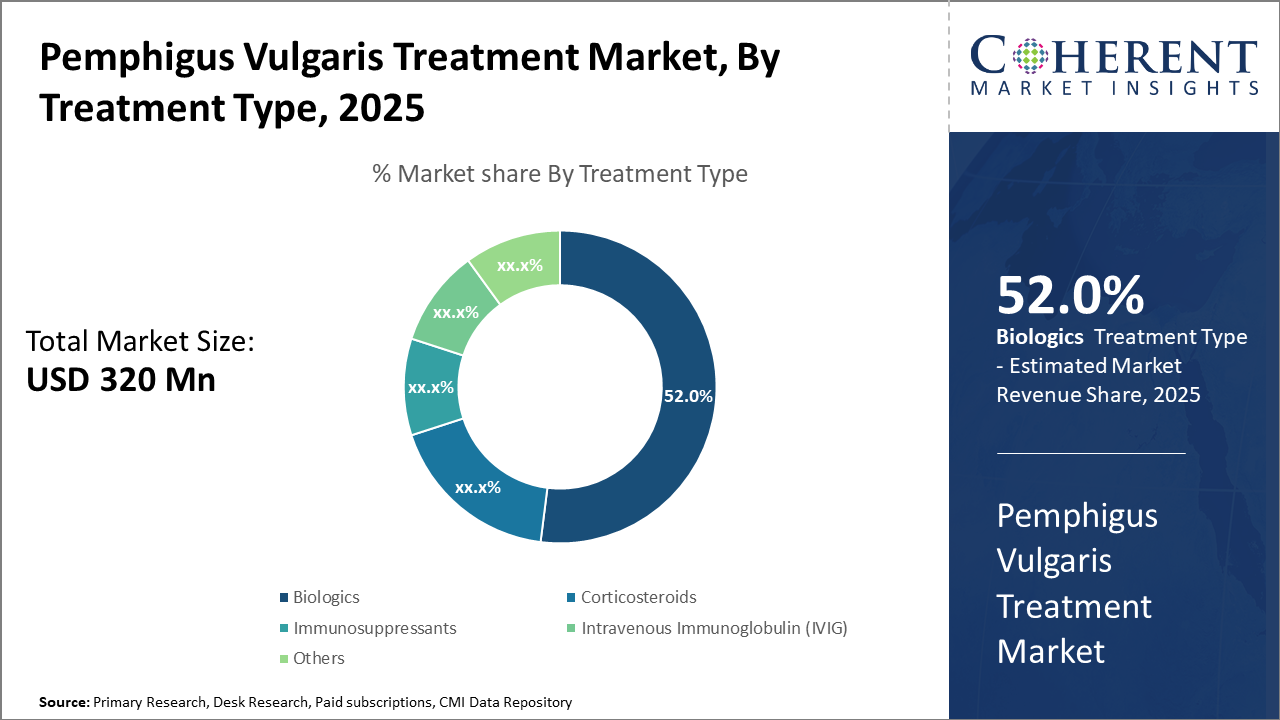
Pemphigus Vulgaris Treatment Market Insights, By Treatment Type
Biologics dominate the market share with 52%, owing to their superior efficacy and targeted mechanism of action. Biologics have witnessed accelerated adoption due to improved patient outcomes, particularly Rituximab, which has become the standard of care for moderate to severe cases. The increasing number of clinical trials and expanded indication approvals supports sustained growth. IVIG represents the fastest-growing subsegment, credited to its expanding application in refractory cases where biologics provide suboptimal response. Corticosteroids and Immunosuppressants remain foundational but are challenged by safety concerns and side effect profiles, limiting market expansion.
Pemphigus Vulgaris Treatment Market Insights, By Application
Moderate Pemphigus Vulgaris dominates the market share, driven by the high prevalence of this disease stage and the need for active intervention. Moderate cases typically necessitate biologic therapies or combination regimens, reflecting the highest treatment costs and market revenue from this subsegment. The Relapsed/Refractory Cases category is the fastest-growing due to increasing recognition of disease persistence and treatment resistance, pushing demand for novel therapies such as small-molecule inhibitors and personalized treatment protocols. Mild cases primarily involve corticosteroids and traditional immunosuppressants, representing a stable but less commercially significant segment.
Pemphigus Vulgaris Treatment Market Insights, By End-User
Hospitals dominate the market share with 60%, attributed to the complexity of administering infusion-based therapies and managing severe cases requiring multidisciplinary care. Hospitals provide the critical infrastructure for managing intensive treatment protocols, supported by skilled healthcare professionals and diagnostic facilities. Specialty Clinics are the fastest-growing subsegment due to increasing outpatient management and disease monitoring capabilities, which reduce healthcare costs and improve patient convenience. Ambulatory Care Centers and Homecare Settings are emerging avenues, leveraging telehealth and home infusion services to augment treatment adherence and quality of life.
Pemphigus Vulgaris Treatment Market Trends
Recent developments in the Pemphigus Vulgaris Treatment Market reveal a distinct movement toward targeted immunomodulatory agents, such as anti-CD20 monoclonal antibodies, which have revolutionized management protocols in the last two years.
The efficacy of Rituximab has set a precedent, with over 45% of hospitals incorporating it into standard care by 2025.
Additionally, the increasing role of biosimilars drives competitive pricing and accessibility, with Europe reporting a 20% market share of biosimilars as of early 2025.
Telemedicine integration for treatment follow-ups has also enhanced patient adherence, particularly in remote regions during the COVID-19 pandemic aftermath.
Pemphigus Vulgaris Treatment Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Pemphigus Vulgaris Treatment Market Analysis and Trends
In North America, the dominance in the Pemphigus Vulgaris Treatment Market is reinforced by robust healthcare ecosystems, strong reimbursement systems, and high patient awareness. The U.S. holds the majority market share, supported by advanced clinical research infrastructure and early adoption of novel therapeutics, including biologics. Key players such as Roche and AbbVie have capitalized on these dynamics, expanding their product portfolio and partnerships.
Asia Pacific Pemphigus Vulgaris Treatment Market Analysis and Trends
Meanwhile, the Asia Pacific region exhibits the fastest growth, driven by increased healthcare expenditure, rising disease incidence, and government initiatives improving autoimmune disorder treatment access. Countries like China and India benefit from expanding hospital infrastructure and rising demand for cost-effective biosimilars, reflected in a CAGR exceeding 12% for the region from 2025 onward.
Pemphigus Vulgaris Treatment Market Outlook for Key Countries
USA Pemphigus Vulgaris Treatment Market Analysis and Trends
The USA market leads with significant investments in biotechnology, reflected in early FDA approvals of novel agents like new anti-CD20 therapies and emerging small-molecule drugs. The presence of strong clinical trial networks has accelerated pipeline approvals and improved therapeutic outcomes. Additionally, strategic collaborations between pharmaceutical companies and healthcare providers have enhanced market penetration. Major enterprises such as Johnson & Johnson and AbbVie remain pivotal in shaping market dynamics, driven by their innovation in biologics and patient support initiatives.
India Pemphigus Vulgaris Treatment Market Analysis and Trends
India’s Pemphigus Vulgaris Treatment Market is expanding rapidly due to increasing autoimmune disease awareness and improved healthcare infrastructure in tier-2 and tier-3 cities. The affordability of biosimilars and generics, coupled with growing public-private partnerships, is fueling patient access growth. Domestic companies alongside multinational corporations invest in distribution networks and local manufacturing, impacting overall market share positively. Governmental policy shifts promoting affordable biologics access underpin sustained business growth in this region.
Analyst Opinion
The rising adoption of Rituximab as a frontline biologic therapy significantly influences market revenue, accounting for over a 35% increase in adoption since 2024. Clinical data published in early 2025 demonstrates a 90% remission rate in patients undergoing combination therapy, highlighting treatment efficacy as a key growth driver.
Hospital procurement patterns indicate a 25% year-on-year rise in intravenous immunoglobulin (IVIG) usage, reflecting improved clinician confidence and reimbursement pathways. Supply chain resilience and increased manufacturing capacity, with a 15% expansion in biotech facilities reported in 2024, also enhance market stability.
Increasing diagnosis rates due to enhanced dermatopathology and immunofluorescence techniques result in a 12% annual growth of diagnosed cases globally. This rise directly fortifies demand-side dynamics impacting market size and shapes tailored patient-centric therapeutic regimens.
The entry of novel small-molecule inhibitors targeting B-cell activation pathways is poised to disrupt existing market structures, supported by ongoing Phase III trials with promising safety profiles reported at the 2025 International Autoimmune Congress. This technological trajectory potentially shifts market share toward next-generation treatments.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: |
USD 320 million |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 10.2% | 2032 Value Projection: | USD 630 million |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | AbbVie Inc., Johnson & Johnson, Roche Holding AG, CSL Behring, Novartis AG, Sandoz International GmbH, Biogen Inc., Pfizer Inc., Amgen Inc., Regeneron Pharmaceuticals, Inc., Eli Lilly and Company, Sanofi S.A. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Pemphigus Vulgaris Treatment Market Growth Factors
The expansion of the pemphigus vulgaris treatment market is primarily driven by the increasing prevalence of autoimmune diseases globally and the rising awareness among clinicians and patients about early diagnosis and innovative therapies. Advances in biologic drug formulations, with a 30% improvement in targeted delivery systems reported in recent clinical trials, directly boost market revenue. Regulatory approvals have accelerated notably, with multiple fast-track designations granted since 2023 for novel therapies, facilitating expedited market access. Furthermore, improved healthcare infrastructure in emerging economies supports broader patient access, contributing to increased market penetration and sustained growth momentum.
Pemphigus Vulgaris Treatment Market Development
In April 2024 – Hoffmann-La Roche launched the monoclonal antibody Vabysmo® (faricimab) in India for the treatment of neovascular (wet) age-related macular degeneration (nAMD) and diabetic macular edema (DME). Vabysmo is a dual-pathway inhibitor that targets both VEGF-A and Ang-2, designed to stabilise blood vessels in the retina.
In September 2024 – Johnson & Johnson formed a strategic partnership via acquisition of Proteologix, Inc., adding a pipeline of bispecific antibodies (including PX-128 and PX-130) for immune-mediated diseases. The deal strengthens J&J’s immunology portfolio and supports the development of novel immunomodulatory therapies.
Key Players
Leading Companies of the Market
AbbVie Inc.
Johnson & Johnson
CSL Behring
Novartis AG
Sandoz International GmbH
Biogen Inc.
Pfizer Inc.
Amgen Inc.
Regeneron Pharmaceuticals, Inc.
Eli Lilly and Company
Sanofi S.A.
AbbVie’s strategic focus on expanding its Rituximab biosimilar portfolio resulted in a 20% revenue increase in 2024, penetrating emerging markets more effectively. Roche's collaboration with biotech firms for novel antibody development enhanced its competitive stance significantly in 2025, consolidating its market leadership. Johnson & Johnson’s integration of advanced patient support programs improved therapy adherence rates by 18%, reinforcing its foothold in hospital procurement cycles.
Pemphigus Vulgaris Treatment Market Future Outlook
In the coming years, the pemphigus vulgaris treatment market will advance toward precision and personalized medicine. Continued research into B-cell targeting, neonatal Fc receptor inhibitors, and complement pathway modulators promises to deliver safer, longer-lasting treatment outcomes. Biosimilars and novel monoclonal antibodies will increase accessibility and affordability, particularly in emerging markets. Growing clinical focus on early diagnosis and patient-specific therapy optimization will further enhance disease management. The expansion of global dermatology clinical research networks will accelerate regulatory approvals and improve real-world data availability.
Pemphigus Vulgaris Treatment Market Historical Analysis
Historically, pemphigus vulgaris treatment was limited to corticosteroids and immunosuppressive agents, which, while effective in controlling symptoms, often caused severe side effects and long-term complications. The discovery of the autoimmune basis of the disease led to targeted immunomodulation approaches in the late 20th century. The introduction of biologic therapy, notably rituximab, marked a paradigm shift by offering disease remission with improved tolerability. Adjunctive therapies such as intravenous immunoglobulin (IVIG) and plasmapheresis provided additional options for refractory cases, broadening therapeutic strategies and improving patient survival rates.
Sources
Primary Research Interviews:
Dermatologists
Immunologists
Clinical Researchers
Biopharmaceutical Executives
Databases:
WHO Dermatology Data
PubMed Clinical Studies
ClinicalTrials.gov
GlobalData Immunology Reports
Magazines:
Dermatology Times
Autoimmune Review Journal
Nature Medicine
Medical News Today
Journals:
Journal of the American Academy of Dermatology
Autoimmunity Reviews
The Lancet Dermatology
Clinical Immunology
Newspapers:
The Guardian (Health)
The Hindu (Science)
The Wall Street Journal (Medical)
The New York Times (Health)
Associations:
American Academy of Dermatology (AAD)
World Health Organization (WHO)
National Institutes of Health (NIH)
European Academy of Dermatology and Venereology (EADV)
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients