The pediatric clinical trials market is estimated to be valued at USD 19.26 Bn in 2025 and is expected to reach USD 37.08 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 9.8% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
The global pediatric clinical trials market is poised for substantial growth, driven by an increasing focus on drug development tailored for pediatric patients and a rising prevalence of chronic diseases among children. This heightened attention to pediatric health necessitates more specialized clinical trials to ensure effective treatments. However, challenges such as regulatory hurdles, ethical considerations, and the need for adequate funding may restrain the pediatric clinical trials industry expansion.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
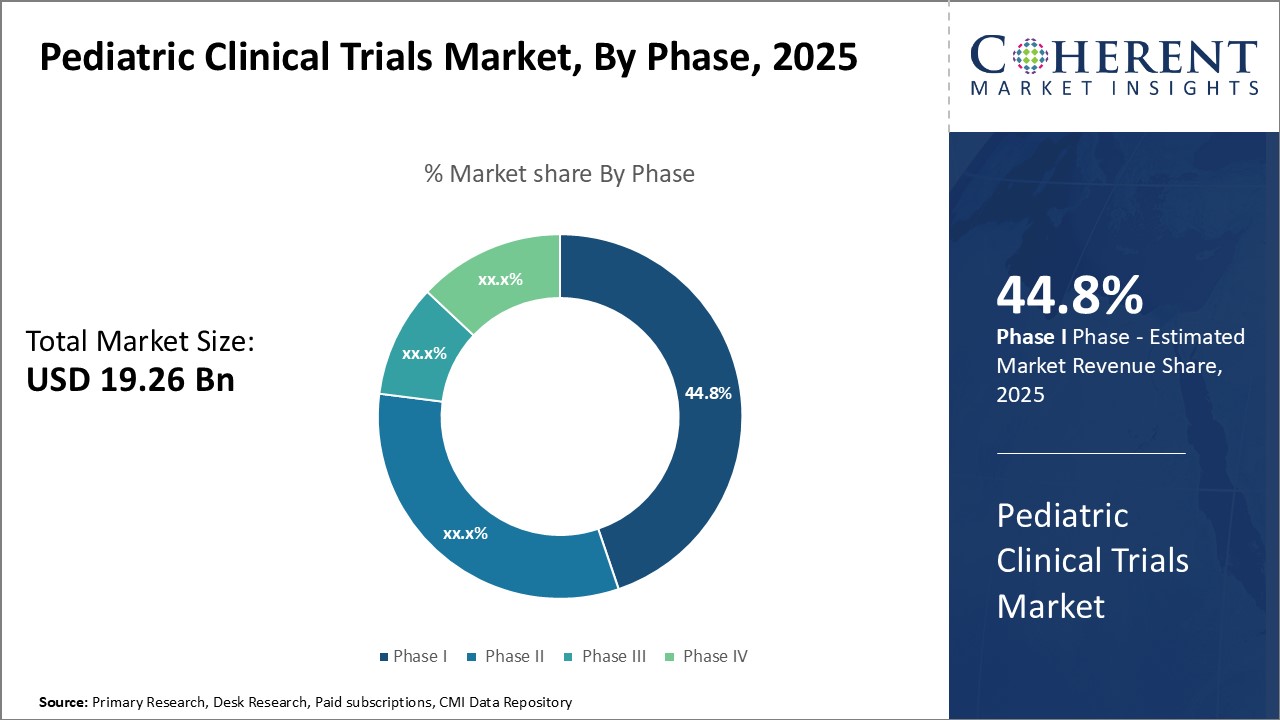
Insights by Phase: Critical role of phase III in drug development drives segment growth
In terms of phase, the phase III segment is expected to contribute the highest market share of 44.8% in 2025 in the pediatric clinical trials industry, owing to its critical role in evaluating drug safety. Phase III trials involve large patient populations, allowing for the comprehensive assessment of a new treatment's effectiveness. This phase involves large patient populations, enabling comprehensive assessments of treatment efficacy and identification of potential side effects.
Insights by Study Design: Emphasis on clinical effectiveness drives treatment studies
In terms of study design, the treatment studies segment is expected to contribute the highest market share of 63.2% in 2025 in the pediatric clinical trials industry, due to stringent requirements from regulatory agencies to demonstrate clinical impact. Treatment studies evaluate the efficacy, effectiveness, and safety of experimental therapies intended to treat or cure a pediatric medical condition. These interventional trials involve administering an investigational product to participants and comparing specific outcomes against standard therapies or placebo controls.
Insights by Therapeutic Area: The respiratory diseases segment dominates due to their high prevalence
By therapeutic area, the respiratory diseases segment is expected to account for the highest market share of 39.7% in 2025 in the pediatric clinical trials industry, owing to the high prevalence and severity of conditions like asthma and respiratory syncytial virus (RSV) infections in children. Respiratory illnesses are a leading cause of pediatric hospitalizations and physician visits globally each year. The establishment of new drugs specifically optimized for pediatric respiratory patients thus represents an area of significant unmet need and commercial potential.
Need a Different Region or Segment? Download Free Sample
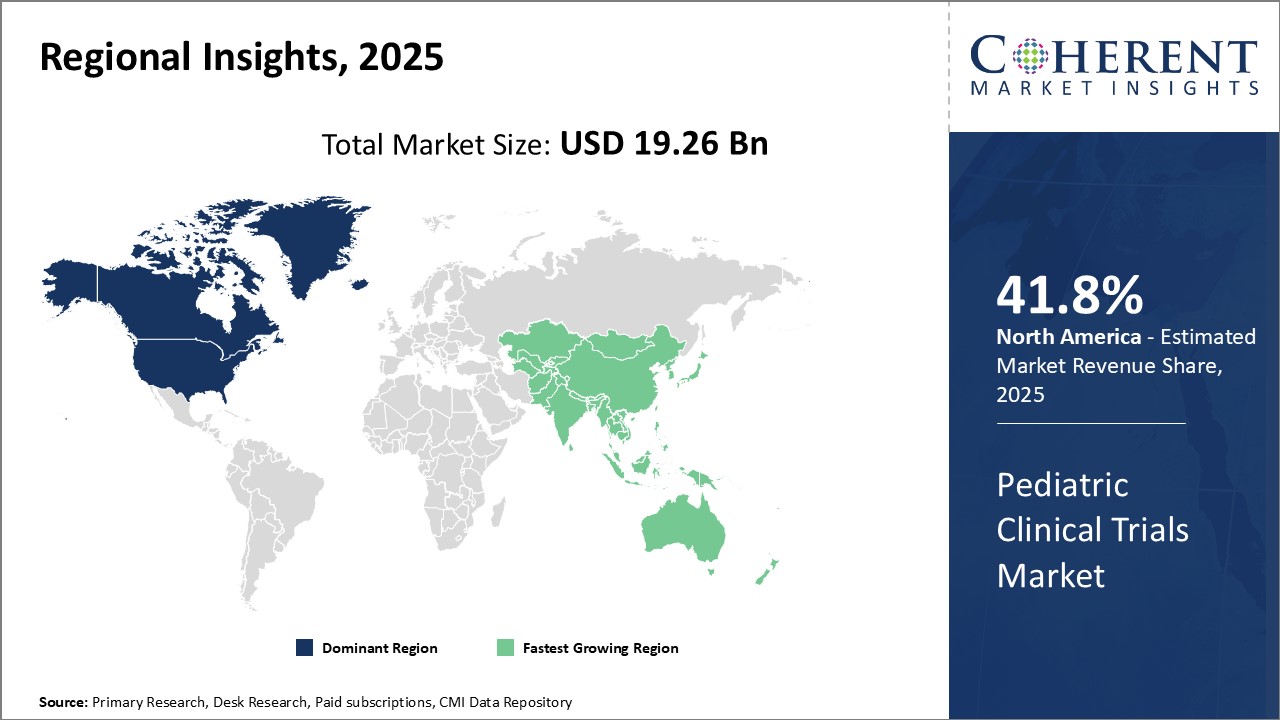
Dominating Region: North America
North America is expected to account for the greatest revenue share of 41.8% in 2025, due to favorable government policies supporting clinical research. Presence of major pharmaceutical companies and clinical research organizations (CROs) have established this region as a leader in clinical research.
Fastest-Growing Region: Asia Pacific
The Asia Pacific region exhibits the fastest growth with 18.9% share in 2025, driven by rising incomes, an expanding patient population, and increased government investments in healthcare and clinical development. Countries also aim to strengthen their pharmaceutical industries and provide affordable drugs for domestic and international market.
Pediatric Clinical Trials Market Outlook for Key Countries
U.S.’ increasing research and development activities
The U.S. pediatric clinical trials industry continues to attract significant investments from sponsors due to its pro-research environment and highly skilled workforce. Major players like Pfizer, Inc. and Eli Lilly and Company conduct extensive trials for pediatric indications from their U.S. bases, leveraging the country's regulatory support and advanced infrastructure. This focus on pediatric research not only addresses the unique medical needs of children but also enhances the development of safe and effective treatments tailored specifically for younger populations.
China’s increasing patient awareness
China pediatric clinical trials industry is poised for significant growth, driven by health reforms, increasing patient awareness, and a focus on developing innovative treatments. Multinational companies are accelerating local clinical activities through partnerships with Chinese firms, enhancing research capabilities. This collaboration not only addresses the unique healthcare needs of children but also fosters a robust environment for pediatric drug development, ultimately improving access to safe and effective therapies for young patients.
India’s favorable cost structure
India continues to lead clinical trial volumes in Asia Pacific, driven by a large treatment-naïve population and a favorable cost structure. This environment enables Indian firms like Dr. Reddy's Laboratories Ltd. and Sun Pharmaceutical Industries Ltd. to pursue global pediatric trial partnerships effectively. Their collaborations are essential for advancing research in pediatric medicine, addressing unmet medical needs, and enhancing access to innovative treatments for children.
Regulatory support in Germany
The pediatric clinical trials market growth in Germany is driven by regulatory support. The European Pediatric Regulation mandates that pharmaceutical companies conduct trials involving children, incentivizing research in this demographic. This regulation aims to enhance the health of children by facilitating the development and availability of pediatric medicines.
Get actionable strategies to beat competition: Download Free Sample
Top Strategies Followed by Pediatric Clinical Trials Market Players
Emerging Startups in the Pediatric Clinical Trials Market
Key Takeaways from Analyst
Pediatric Clinical Trials Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 19.26 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.8% | 2032 Value Projection: | USD 37.08 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
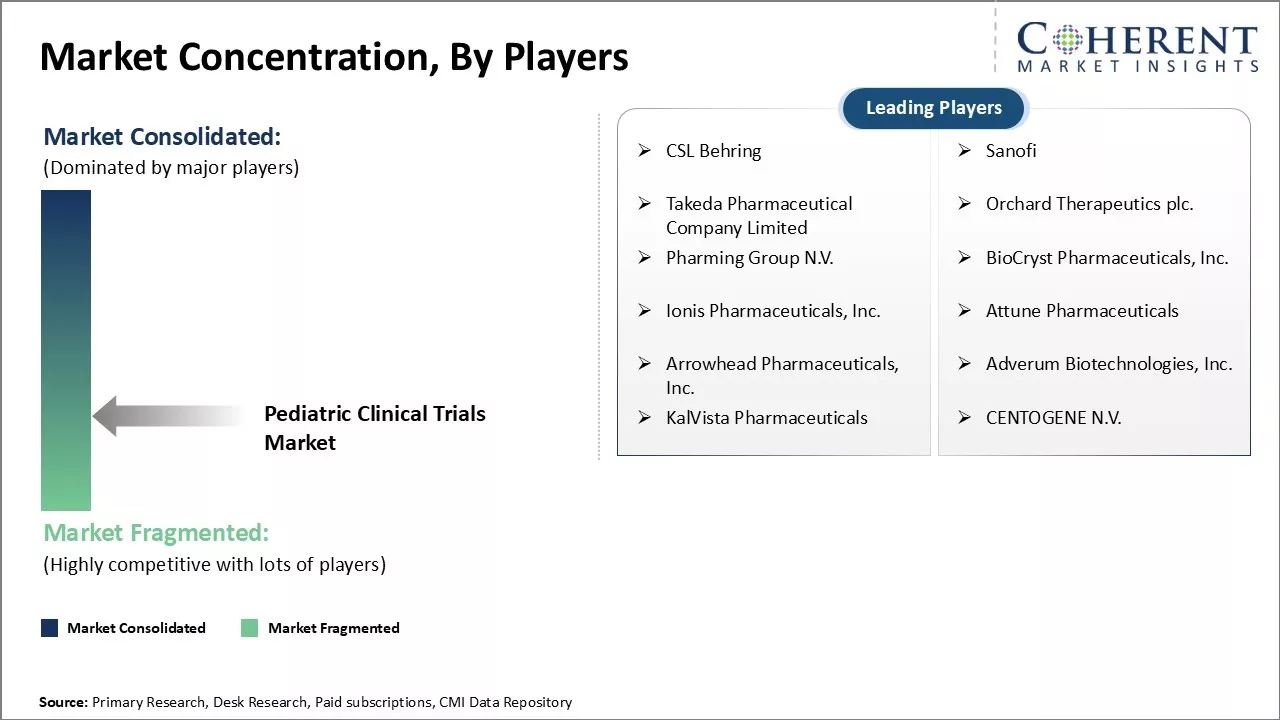
| Companies covered: |
CSL Behring, Sanofi, Takeda Pharmaceutical Company Limited, Orchard Therapeutics plc., Pharming Group N.V., BioCryst Pharmaceuticals, Inc., Ionis Pharmaceuticals, Inc., Attune Pharmaceuticals, Arrowhead Pharmaceuticals, Inc., Adverum Biotechnologies, Inc., KalVista Pharmaceuticals, and CENTOGENE N.V. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
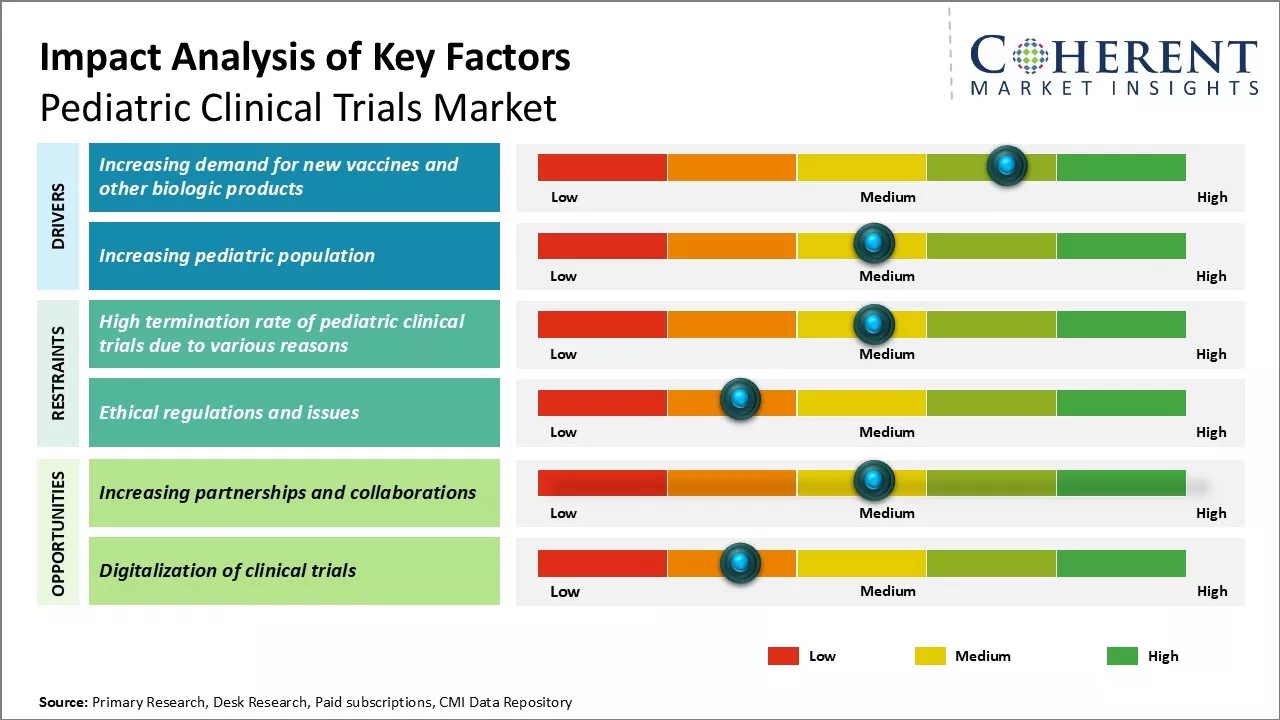
Market Driver - Increasing demand for new vaccines and other biologic products
The development of new vaccines and biological products for children has been increasing, focusing on preventing infectious diseases and improving health outcomes which drives the pediatric clinical trials industry growth. Pharmaceutical companies are investing more in their pediatric pipelines, recognizing the commercial potential. While vaccines have successfully controlled many childhood illnesses, diseases like respiratory syncytial virus (RSV) and group B streptococcus (GBS) still pose risks, prompting ongoing research for effective candidates.
Market Challenge - High termination rate of pediatric clinical trials due to various reasons
One major challenge in the pediatric clinical trials market is the high termination rate. Factors contributing to this include difficulties in patient enrollment due to a smaller target population, stringent safety standards, ethical concerns, and high dropout rates. Additionally, insufficient funding deters pharmaceutical companies from investing in pediatric research, while complex regulations and operational hurdles extend timelines and increase costs, prompting sponsors to abandon trials midway.
Market Opportunity - Increasing Partnerships and Collaborations
One of the major opportunities in the pediatric clinical trials market is the increasing partnerships and collaborations between various stakeholders. For instance, in February 2023, MMS Holdings Inc., a pharmaceutical company, announced a partnership with the Institute for Advanced Clinical Trials (I-ACT) to accelerate the development of life-saving therapeutics including vaccines, medicines, and medical devices for children.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients