Global PARP inhibitor market is estimated to be valued at USD 7.85 Bn in 2025 and is expected to reach USD 14.36 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 9.0% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
Increasing prevalence of cancer including breast and ovarian cancer can drive the market growth. PARP inhibitors are emerging as an important class of targeted therapeutics for the treatment of various types of cancers associated with BRCA1 and BRCA2 gene mutations. Many clinical trials are being conducted to evaluate the efficacy of PARP inhibitors against other cancer indications beyond ovarian cancer. Moreover, increasing awareness about cancer treatment options and gaining popularity of personalized medicine approach can also drive the market growth.
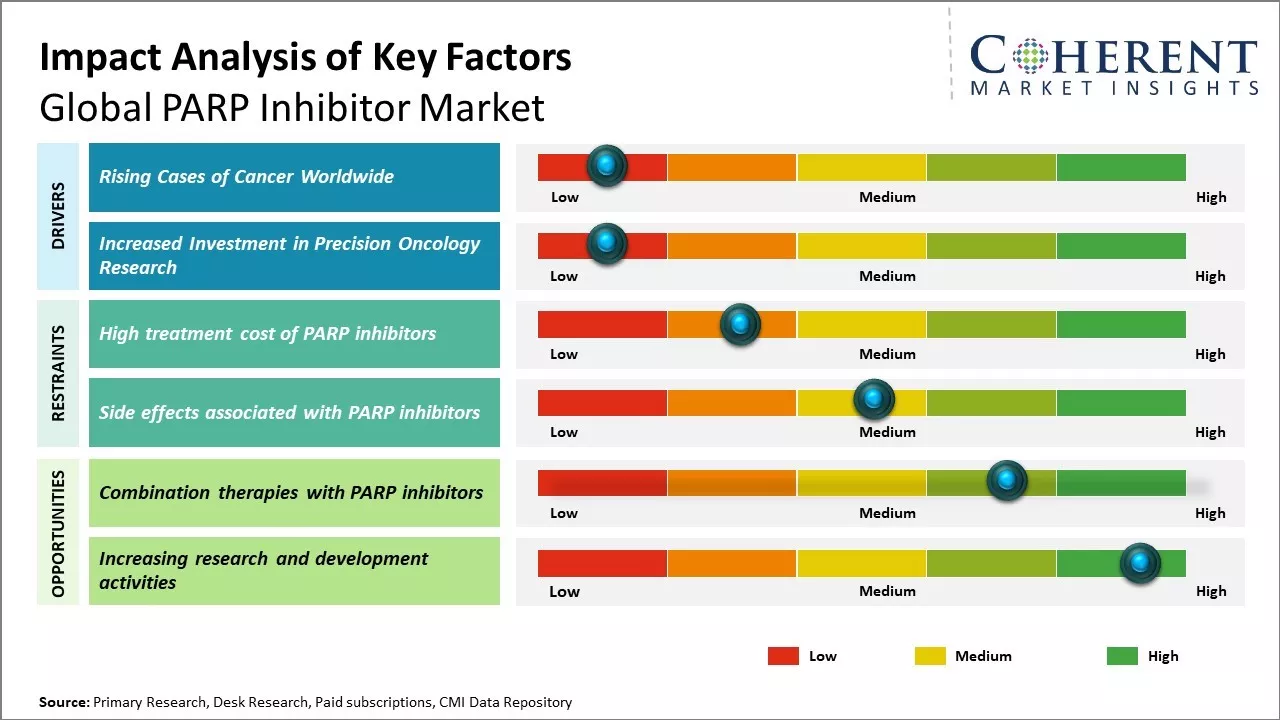
Rising Cases of Cancer Worldwide
Global PARP inhibitor market growth is driven by rising prevalence of cancer around the world. Cancer has become one of the leading causes of mortality with an estimated 9.6 million deaths in 2018. Growing geriatric population can also increase the burden of cancer worldwide. As per estimates, the population aged 60 years and above is expected to double from 12% to 22% between 2015 and 2050. With age being one of the primary risk factors for cancer, the rising global elderly demographic can pose a major public health challenge. Furthermore, improvements in diagnostic capabilities and screening programs have led to enhanced detection rates of various malignancies in recent years. Developed economies have witnessed major transformations in cancer screening with widespread adoption of advanced diagnostic technologies. This has resulted in earlier disease detection and increased diagnosis rates. However, this has also expanded the pool of patients eligible for targeted therapies such as PARP inhibitors. PARP inhibitors have been demonstrated to be effective for certain patients suffering from recurrent ovarian and breast cancers having mutations in homologous recombination repair (HRR) genes like BRCA1 or BRCA2. As more patients get diagnosed at treatable stages, there has been demand for precision oncology drugs like PARP inhibitors for long-term management of cancer. For instance, in February 2025, according to World Health Organization, the number of cancer cases worldwide is rising, revealing big differences in how people are treated and what happens to them afterward. Lung, breast, and colorectal cancers are the most common types. In the future, experts predict a 77% increase in cases by 2050.
Get actionable strategies to beat competition: Download Free Sample
Increased Investment in Precision Oncology Research
There has been a paradigm shift towards targeted and personalized treatment approaches in oncology that has led to significant investments from biopharmaceutical companies, governments, and private foundations to support precision oncology research. Large drug makers have been prioritizing investment in the development of tumor-agnostic as well as tumor-specific targeted therapies. Rapid advancements are being made through immuno-oncology and molecularly targeted agents, including PARP inhibitors, that have enabled treatment of cancers based on their molecular profile rather than site of origin. With improved understanding of cancer genomics and biomolecular underpinnings of the disease, PARP inhibitors are being evaluated across various tumor types harboring defects in DNA repair mechanisms. Research exploring new indications and combination regimens has expanded the use of currently approved PARP inhibitors. This has also aided pharmaceutical companies in maximizing returns on their drug development investments. Several small biotechs are involved in advancing innovative therapeutic candidates in their pipelines. Governments and charities are also making efforts to address gaps in precision cancer research through funding initiatives.
Key Takeaways from Analyst:
PARP inhibitors have rapidly emerged as an important therapeutic option for certain cancers with DNA repair deficiencies. The approval of Lynparza and Zejula to treat later-line ovarian cancer and supportive data across tumor types indicate that PARP inhibitors can provide meaningful clinical benefits to patients.
North America currently dominates the PARP inhibitor market due to early FDA approvals and strong reimbursement structures supportive of innovative oncology therapies. However, Europe is also a major market due to centralized approval process and universal healthcare. The Asia Pacific region is poised to experience the fastest growth due to rising healthcare investments, an increasing cancer burden, and expanding health insurance coverage across key markets like China and India.
High treatment costs and narrow window of therapeutic activity can hamper the market growth. Ongoing trials evaluating PARP inhibitors for additional tumor types and earlier lines of therapy can expand the eligible patient pool and drive broader uptake of these inhibitors. Partnerships between pharma companies may facilitate widening investigator-initiated study portfolios.
Though generic competition threatens the likes of Lynparza in the long run once patents expire, subsequent market entrants are introducing new PARP inhibitors with differing profiles that could carve out meaningful shares.
Market Challenges: High treatment cost of PARP inhibitors
The exorbitant cost of PARP inhibitor drugs can hamper the global PARP inhibitor market growth. PARP inhibitors have proven to be effective therapeutic options for certain cancers like ovarian and breast, however, the astronomical cost of treatment makes it inaccessible to many patients. For instance, the cost of one year of Lynparza treatment ranges between US$ 100,000 to US$ 150,000 in the U.S., according to a study conducted by the National Comprehensive Cancer Network (NCCN) in 2021. Zejula treatment costs about US$ 136,000 per year. Such high costs place an enormous financial burden on patients as well as national health budgets. Many developed countries like the U.S. do not have universal healthcare and patients are required to bear a major part of the treatment expenditure through out-of-pocket payments. This deters patients from opting for PARP inhibitor therapy and increases the rate of treatment discontinuation. The situation is worse in developing and underdeveloped countries where a sizable population lacks health insurance. As per data from the World Bank, over half the population in India does not have health coverage. This makes life-saving cancer treatments like PARP inhibitors completely unaffordable for most patients in the country. The high costs of PARP inhibitors diminish their availability globally. Pharmaceutical companies are also hesitant to invest in research and development of new PARP inhibitors due to uncertainty over cost recovery. Unless measures are taken to make these drugs more affordable and accessible, the PARP inhibitor market may fail to realize its projected growth rates over the next few years.
Market Opportunities: Combination therapies with PARP inhibitors
Combination therapies with PARP inhibitors offer promising opportunities for the global PARP inhibitor market growth. Recent clinical trials have shown positive results of combining PARP inhibitors with other anti-cancer drugs for treating various cancers. For example, in 2020, according to the study published by Lancet Oncology Journal, improved progression-free survival in women with ovarian cancer when niraparib (a PARP inhibitor) was combined with bevacizumab (an anti-VEGF drug) compared to niraparib alone. As monotherapy resistance to PARP inhibitors remains a challenge in breast and prostate cancers, combination regimens are being actively explored. Several ongoing late-phase clinical trials are evaluating PARP inhibitors in combination with immunotherapy drugs like PD-1/PD-L1 inhibitors for breast, ovarian and prostate cancers. Preliminary results have indicated combination therapy may help overcome resistance and significantly improve treatment outcomes. For instance, a Phase 3 trial presented at the 2021 European Society for Medical Oncology Congress showed improved survival when olaparib was combined with immunotherapy versus standard of care for metastatic prostate cancer with BRCA mutations. As such combination regimens gain regulatory approvals and are incorporated into treatment guidelines based on positive trial results, it can significantly expand the eligible patient pool for PARP inhibitors. This will can boost demand. According to the National Cancer Institute, the number of new cancer cases per year is expected to rise to over 30 million by 2040 worldwide due to growth and aging of the population. The increasing cancer incidence coupled with advancement of targeted combination therapies can offer opportunity for players in the PARP inhibitor market.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
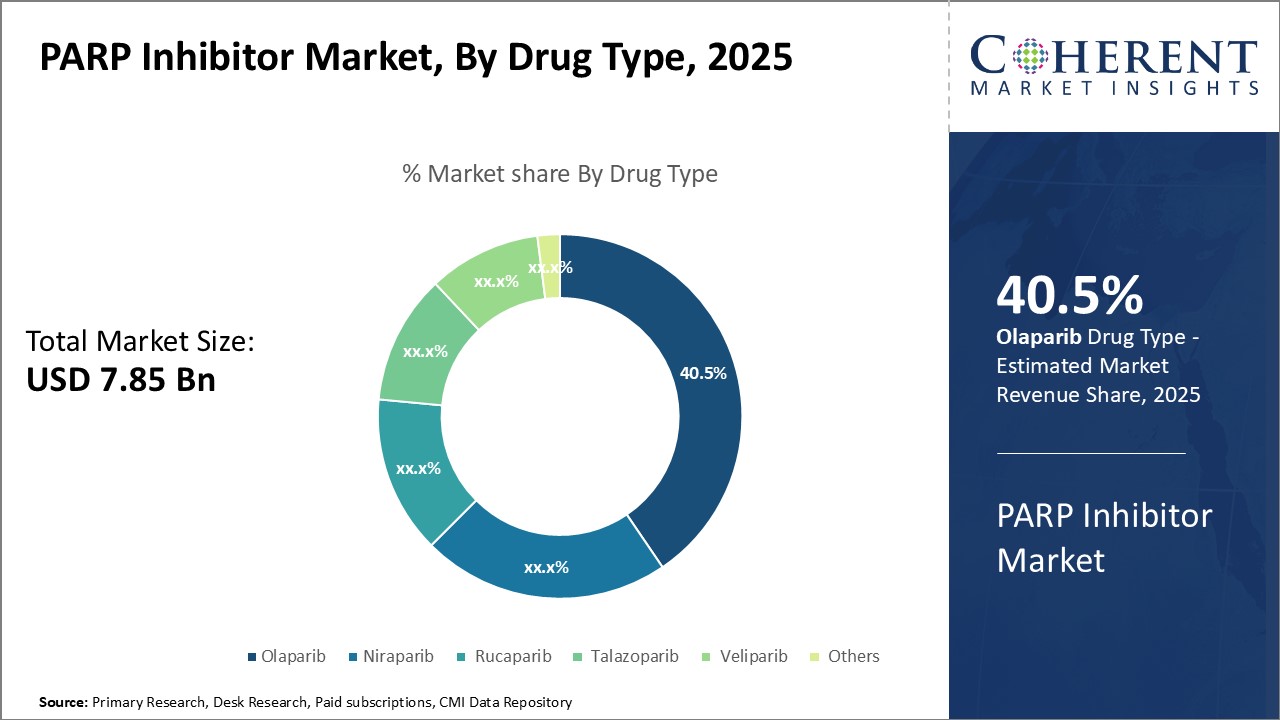
By Drug Type- Driving Acceptance and Affordability, Olaparib contributes the highest share of the market
In terms of drug type, olaparib segment is estimated to contribute the highest market share of 40.5% in 2025, owing to its leading efficacy and acceptable side effect profile. With a well-established safety and effectiveness profile demonstrated in numerous clinical trials, Olaparib has become the standard-of-care PARP inhibitor for certain breast and ovarian cancer patients. Its oral administration also provides convenience over intravenous alternatives. Growing affordability of Olaparib as several generics have recently entered the market post-patent expiry has boosted its adoption rates. This has particularly benefitted patients in price-sensitive developing nations.
By Application- Ovarian Cancer contributes the highest share of the market due to its rising prevalence
In terms of application, ovarian cancer segment is estimated to contribute the highest market share of 30.5% in 2025, owing to its rising prevalence globally as well as greater receptiveness to PARP inhibitor treatments. Ovarian cancer is among the deadliest gynecological cancers with symptoms often appearing at later stages, necessitating more invasive treatments. PARP inhibitors have emerged as an important maintenance therapy after frontline treatment showing significant gains in progression-free and overall survival for ovarian cancer patients. With aging populations in most regions, there has been increasing incidence of ovarian cancer that boosts demand for PARP inhibitors.
By Distribution Channel- Hospital Pharmacies contributes the highest share of the market
In terms of distribution channel, hospital pharmacies segment is estimated to contribute the highest market share of 40.5% in 2025, owing to these venues offering convenient access to PARP inhibitors, especially in developing nations with growing disease burden. Hospital pharmacies allow for direct distribution of medication to treating oncologists along with cold storage and logistics support for therapies often requiring preservation. This expands the reach and affordability of PARP inhibitors to a wider patient pool via coverage under public insurance programs. With healthcare infrastructure strengthening globally, the availability of PARP inhibitors through hospital networks is anticipated to grow substantially, further cementing their position as the dominant distribution channel.
Need a Different Region or Segment? Download Free Sample
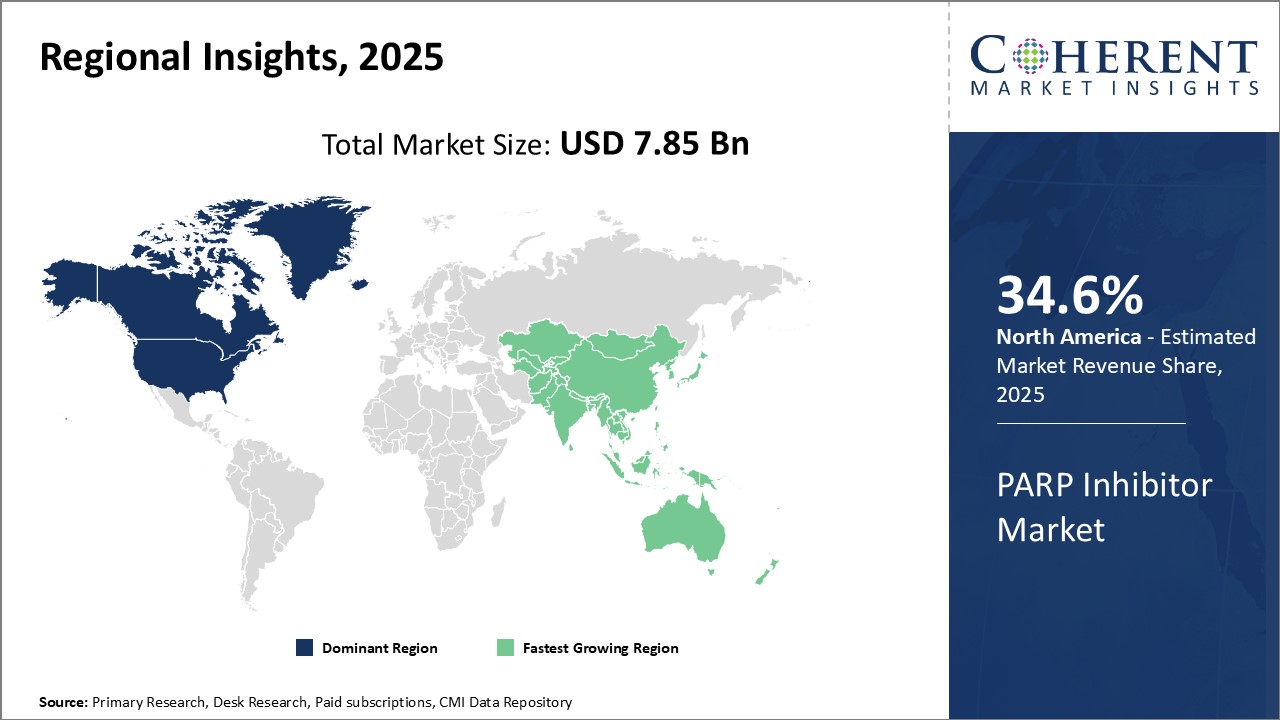
North America dominates the global PARP inhibitor market with an estimated market share of 34.6% in 2025, due to strong presence of leading pharmaceutical companies in the U.S. and Canada that are actively involved in the research and development of innovative PARP inhibitors. Companies like AstraZeneca, Tesaro, Clovis Oncology and Johnson & Johnson have their headquarters in the region. Furthermore, the availability of favorable reimbursement policies for cancer drugs and growing adoption of precision medicine have supported the uptake of PARP inhibitors in the treatment of cancers like ovarian and breast.
However, Asia Pacific region is poised to be the fastest growing market due to rising burden of cancer, increasing healthcare investments by various governments and expansion strategies adopted by key market players. China, Japan and India represent the most lucrative market opportunities due to their large patient pools. Additionally, new manufacturing facilities set up by global drugmakers to cater to the demand from the Asia's emerging economies have made PARP inhibitors more affordable. This has positively influenced their usage across major hospitals as well as small private clinics.
PARP Inhibitor Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 7.85 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.0% | 2032 Value Projection: | USD 14.36 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
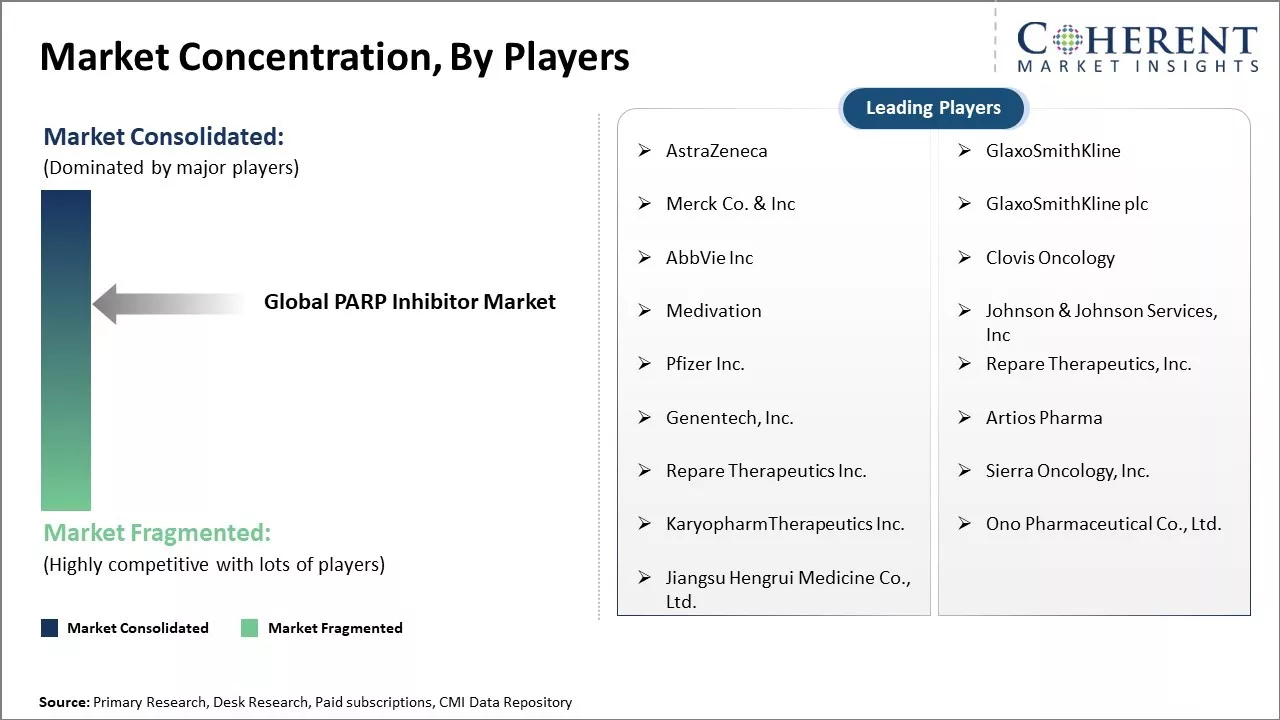
AstraZeneca, GlaxoSmithKline, Merck Co. & Inc, GlaxoSmithKline plc, AbbVie Inc, Clovis Oncology, Medivation, Johnson & Johnson Services, Inc, Pfizer Inc., Repare Therapeutics, Inc., Genentech, Inc., Artios Pharma, Repare Therapeutics Inc., Sierra Oncology, Inc., KaryopharmTherapeutics Inc., Ono Pharmaceutical Co., Ltd., Jiangsu Hengrui Medicine Co., Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: T Global PARP Inhibitor Market involves drugs that target a DNA repair enzyme called poly (ADP-ribose) polymerase or PARP. PARP inhibitors work by blocking cancer cells' ability to repair double-stranded DNA breaks, which helps chemotherapy and radiation kill cancer cells more effectively. They are primarily used for certain types of breast, ovarian and prostate cancers with specific genetic mutations. The global market covers research and development of new PARP inhibitors as well as sales of approved drugs in this class worldwide.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients