Parp Inhibitor Biomarkers Market is estimated to be valued at USD 1,414.9 Mn in 2025 and is expected to reach USD 6,709.1 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 24.9% from 2025 to 2032.
Analysts’ Views on Global PARP Inhibitor Biomarkers Market:
Increasing prevalence of Cancer disease, rising geratic population and increasing awareness about Cancer and new Biomarkers releases and strategic efforts by prominent market competitors are favorably impacting the industry's growth. the high prevalence of Cancer, favorable health reimbursement, and increased awareness regarding Cancer among people and clinicians is a major factor in the PARP inhibitor biomarkers market, thereby contributing to the market growth.
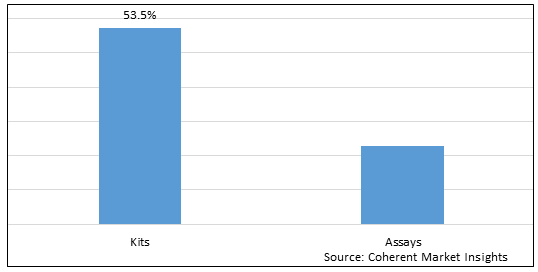
Figure 1. Global PARP Inhibitor Biomarkers Market Share (%),By Product Type, 2025
To learn more about this report, Download Free Sample
Global PARP Inhibitor Biomarkers Market – Driver
Increasing prevalence of Cancer
Increasing prevalence of Cancer is expected to propel growth of the global PARP inhibitor biomarkers market over the forecast period. For instance, according to the article published by the World Health Organization, in February 2022, the age-standardized point prevalence and annual incidence rates of Cancer were 246.6 and 14.9 in 2019, which increased by 7.4% and 8.2% from 1990, respectively. Cancer is a leading cause of death worldwide, accounting for nearly 10 million deaths in 2020.
Increasing expenditure for the treatment and management of Cancer
Increasing expenditure for the treatment and management of of also expected to aid in growth of the market. For instance, according to the article published in the journal of lancet redgional health in October 2022, reported catastrophic expenditure and treatment attrition in patients seeking comprehensive colorectal cancer treatment in India. 226 patients included, 20 died within six months of being offered a treatment plan and four were lost to follow-up. The median total cost of colorectal cancer treatment was 407,508 Indian Rupees (INR/5340 USD), to which the biggest contributor was the patient's PARP (median 330,277 INR/4328 USD).
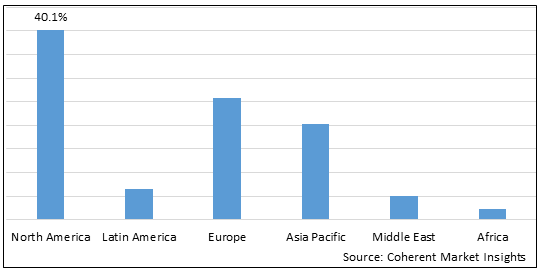
Figure 2. Global PARP Inhibitor Biomarkers Market Value (US$ Million), by Region, 2025
To learn more about this report, Download Free Sample
Global PARP Inhibitor Biomarkers Market- Regional Analysis
Among regions, North America is estimated to hold a dominant position in the global PARP inhibitor biomarkers market over the forecast period. North America is estimated to hold 40.1 % of the market share in 2025. The global PARP inhibitor biomarkers market is expected to witness significant growth in the coming years, driven by the high prevalence of Cancer, favorable health reimbursement, and increased awareness, North America to emerge as the leading region for the PARP Inhibitor Biomarkers market. For instance, according to the press release in February 2020, by imaware, provides laboratory testing for wellness monitoring, informational, and educational use in North America, announced the launch of the At-Home Cancer Screening Test, designed by healthcare company Micro drop. The imaware test for Cancer uses just a few drops of blood to detect unique biomarkers as well as two additional biomarkers to provide more comprehensive results. These kinds of developments in the healthcare system and rising health expenditures, as well as increased knowledge of advanced Cancer treatments, are fueling the Cancer market in North America.
Global PARP Inhibitor Biomarkers Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others are facing problems with the transportation of drugs from one place to another.
However, the COVID-19 pandemic had a positive impact on the global PARP Inhibitor Biomarkers market. The COVID-19 outbreak affected the market's growth adversely in its preliminary phase; however, the number of Cancer patients increased, and so has the demand for the Cancer diagnostic test market. For instance, according to the article published in February 2022 on PubMed Central, “COVID-19 and Cancer Cross talk: Emerging Association, Therapeutic Options and Challenges” explains that the management of Cancer patients in the COVID-19 context is a difficult task in and of itself. Even though online consultations are advisable with patients who are having stable Cancer.
PARP Inhibitor Biomarkers Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1,414.9 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 24.9% | 2032 Value Projection: | USD 6,709.1 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Myriad Genetics, Inc., F. Hoffmann-La Roche AG, Invitae Corporation, NeoGenomics Laboratories, Inc., BPS Bioscience, Inc. Antibodies Inc., Networks LLC, Beckman Coulter, Inc., Euro Diagnostica AB, F. Hoffmann-La Roche Ltd., Qiagen NV, Siemens Healthcare GmbH, Bio Rad Laboratories Inc., Exagen Inc., Genway Biotech, Inc., Microdrop LLC (imaware), Svar Life Science AB and Thermo Fisher Scientific Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global PARP Inhibitor Biomarkers Market Segmentation:
The Global PARP Inhibitor Biomarkers Market report is segmented into by Test Type, By End User and Region.
By Product Type , the market is segmented into Kits and tests out of which, the into Kits is expected to hold a dominant position in the global PARP Inhibitor Biomarkers market during the forecast period and this is attributed to awareness of Cancer Disease.
By Service Type, the market is segmented into BRCA 1 & 2 Testing, HRD Testing, HRR Testing and Others. out of which, the BRCA 1 & 2 Testing is expected to dominate the market over the forecast period and this is attributed to the early tests and prompt treatment can lead to proper recovery.
By Application, the market is segmented into Breast Cancer, Ovarian Cancer, and Others (Prostate, Pancreatic, etc.). Out of which, Breast Cancer Segment is expected to dominate the market over the forecast period and this is attributed to the increases with age and peaks at menopause.
Among all the segmentations, the By Application segment expected to dominate the market over the forecast period and this is attributed to the increasing obesity after menopause is which is expected boost the growth of PARP inhibitor biomarkers market over the forecast period.
Global PARP Inhibitor Biomarkers Market Cross Sectional Analysis:
Key players are making PARP Inhibitor Biomarkers with advance technology emerging economies is also expected to boost demand PARP Inhibitor Biomarkers market in North America region. For instance, in October 2020, Myriad Genetic Laboratories, Inc., an genetic testing and precision medicine company based in Salt Lake City, Utah, U.S., introduced Myriad's BRAC Analysis Treatment method from the Japanese Ministry of Health, Labor, and Welfare to be utilized as a partnered treatment with the PARP constraints.
Global PARP Inhibitor Biomarkers Market: Key Developments
On June 1, 2023, Myriad Genetics, Inc., is a company of genetic testing and precision medicine, launching new studies and expansion of its Precise Oncology Solutions portfolio at the 2023 American Society of Clinical Oncology (ASCO).
In May 2022, United Rheumatology, a rheumatology care management organization empowering rheumatologists to advance the standard of care and education, advocacy, and research organization for people living with Cancer disease, announced a partnership to develop the Cancer Wellness Center, a digital resource aimed at aiding those living with Cancer.
On March 22, 2023, F. Hoffmann-La Roche Ltd (Roche), a biotechnology company that develops drugs and diagnostics to treat major diseases, announced that it has entered into a collaboration with Eli Lilly and Company to support the development of Roche’s Elecsys PARP Inhibitor Biomarkers The PARP is an innovative blood test that aims to facilitate the earlier diagnosis of Alzheimer’s disease.
In March 2022, Invitae, a medical genetics company, announced full access to its Personalized Cancer Monitoring (PCMTM) platform to help detect minimal or molecular residual disease (MRD) in patients with solid tumors. Invitae PCM uses a novel set of personalized assays based on a patient's tumor to detect circulating tumor DNA (ctDNA) in blood, offering the ability to perform risk stratification, response assessment to treatment and detection of cancer recurrence, based on recent studies.
Global PARP Inhibitor Biomarkers Market: Key Trends
Application of biomarker test for diagnosing Cancer
Application of biomarker test for diagnosing Cancer is at an early stage of development. Major players in the market are focused on adopting partnership strategies to expand their product portfolio. Presently, there is no biochemical test available for the diagnosis of cancer. However, Rheumatoid Factor (RF) and anti–cyclic citrullinated peptide (anti–CCP) antibodies test require more refinement to improve their clinical utility. For instance, in March 2020, WorldCare Clinical, LLC, an independent contract research organization offering imaging in clinical trials, partnered with Navidea Biopharmaceuticals, Inc. is a biopharmaceutical company focused on the development of precision immunodiagnostic agents and immunotherapeutics, to offer imaging service following U.S. FDA approval of Navidea’s cancer diagnostic. Navidea’s strategy is to deliver novel products and advancing the Company’s pipeline through global partnering with WorldCare Clinical, LLC.
Global PARP Inhibitor Biomarkers Market: Restraint
High cost of PARP inhibitor biomarker test kits and assays
High cost of PARP inhibitor biomarker test kits and assays is expected to hamper the growth of the global PARP inhibitor biomarkers market. For instance, in May 2020, Myriad Genetics, Inc.’s myChoice CDx became commercially available with a list price of US$ 4,590. myChoice CDx is the FDA has approved myChoice CDx for use as a companion diagnostic to identify patients with advanced ovarian cancer with positive homologous recombination deficiency (HRD) status by detecting BRCA1 and BRCA2.
Global PARP Inhibitor Biomarkers Market - Key Players
Major players operating in the global PARP Inhibitor Biomarkers market include Myriad Genetics, Inc., F. Hoffmann-La Roche AG, Invitae Corporation, NeoGenomics Laboratories, Inc., and BPS Bioscience, Inc. Antibodies Inc., Networks LLC, Beckman Coulter, Inc., Euro Diagnostica AB, F. Hoffmann-La Roche Ltd., Qiagen NV, Siemens Healthcare GmbH, Bio Rad Laboratories Inc., Exagen Inc.,Genway Biotech, Inc., Microdrop LLC (imaware), Svar Life Science AB and Thermo Fisher Scientific Inc.
*Definition: A substance that blocks an enzyme in cells called PARP. PARP helps repair DNA when it becomes damaged. DNA damage may be caused by many things, including exposure to UV light, radiation, certain anticancer drugs, or other substances in the environment. In cancer treatment, blocking PARP may help keep cancer cells from repairing their damaged DNA, causing them to die. PARP inhibitors are a type of targeted therapy, also called as poly (ADP-ribose) polymerase inhibitor.
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients