Paroxysmal Nocturnal Hemoglobinuria Therapeutics Market Size and Forecast – 2025 – 2032
The Global Paroxysmal Nocturnal Hemoglobinuria Therapeutics Market size is estimated to be valued at USD 1.2 billion in 2025 and is expected to reach USD 2.6 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 11.5% from 2025 to 2032.
Global Paroxysmal Nocturnal Hemoglobinuria Therapeutics Market Overview
PNH therapeutics are specialized treatments that target complement-mediated red blood cell destruction, a hallmark of the rare hematologic disorder. Historically, management was limited to supportive therapies such as blood transfusions and corticosteroids, offering minimal disease control. The advent of complement inhibitors, particularly eculizumab, marked a breakthrough by directly targeting the C5 protein to prevent hemolysis. Recent innovations include long-acting formulations like ravulizumab and emerging oral therapies targeting proximal complement pathways for improved convenience and compliance. The development of gene therapies and small molecule inhibitors represents the next phase of progress, aiming for durable remission and reduced treatment frequency.
Key Takeaways
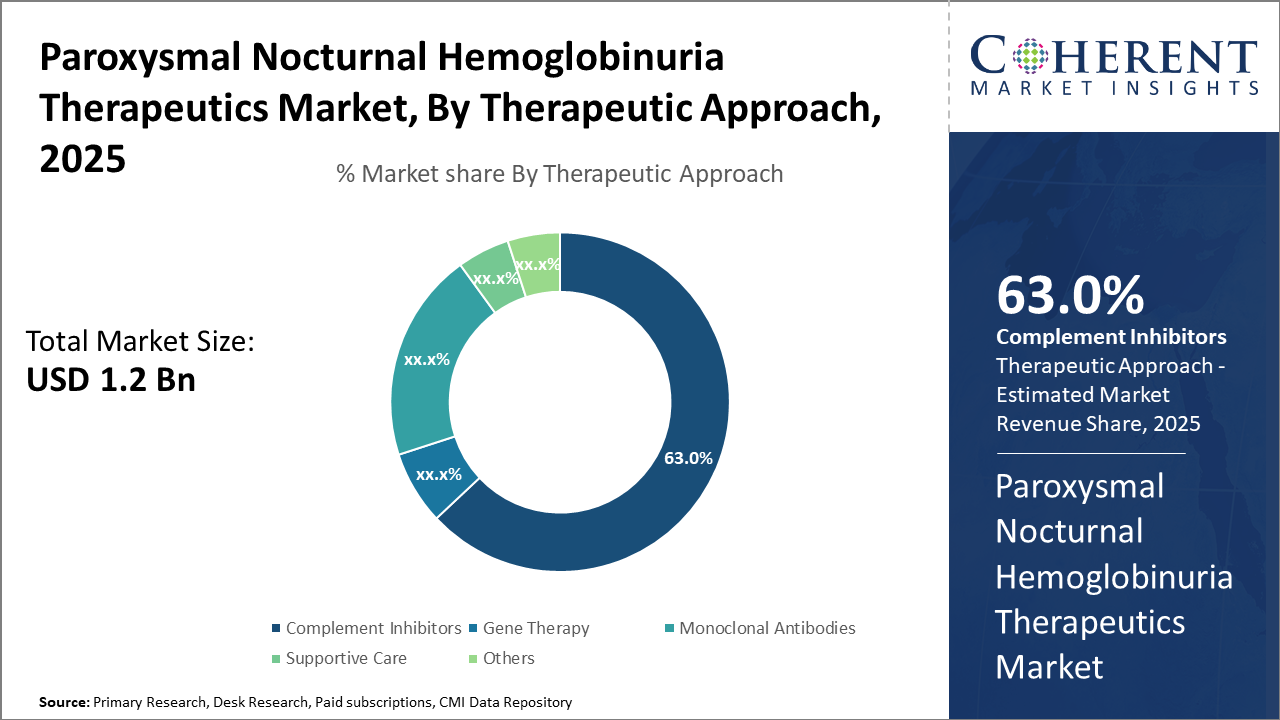
The Complement Inhibitors segment continues to dominate, holding a market share of 63%, driven by clinical efficacy and broader physician acceptance.
The Hospital Pharmacy distribution channel accounts for over 50% of market revenue, reflecting the centralized administration paradigm for PNH therapeutics, although online pharmacy channels are rapidly gaining traction.
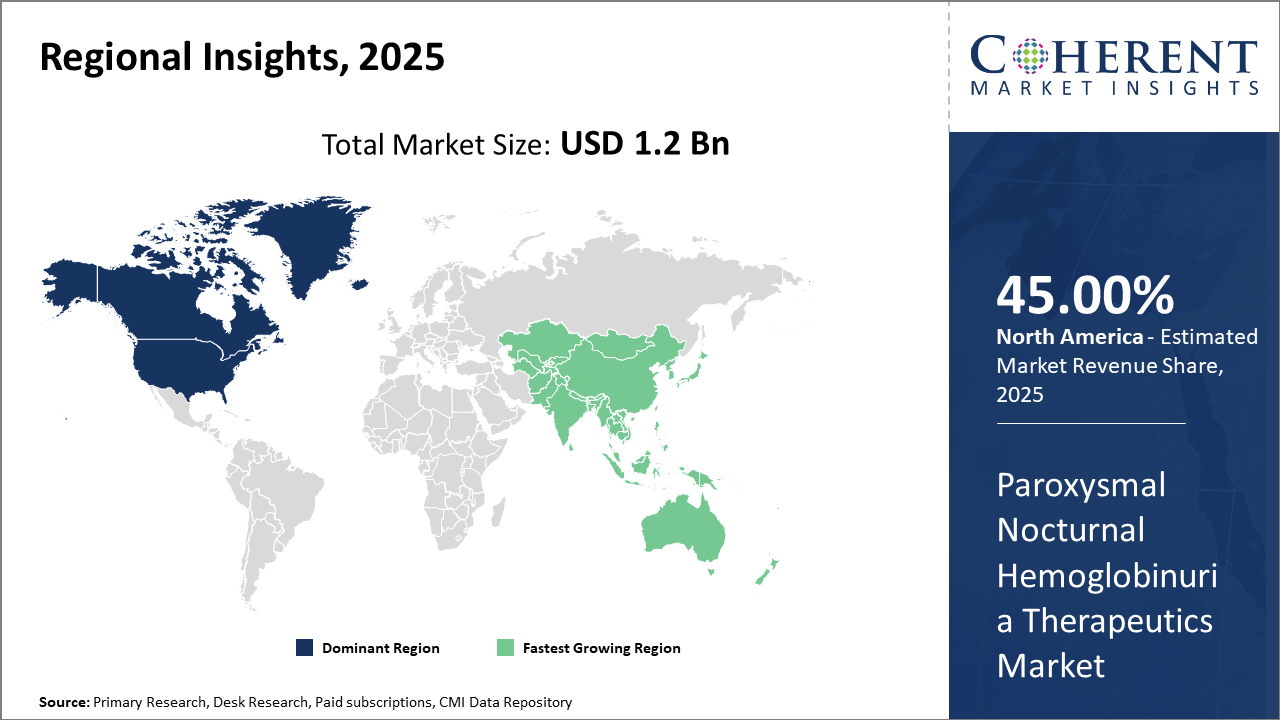
North America maintains dominance in market share at approximately 45%, supported by a robust healthcare ecosystem and regulatory environment conducive to innovation.
Asia Pacific, with high CAGR projections, leverages government initiatives to improve rare disease diagnosis and local manufacturing partnerships, positioning it as the fastest-growing region in the Paroxysmal Nocturnal Hemoglobinuria Therapeutics market.
Paroxysmal Nocturnal Hemoglobinuria Therapeutics Market Segmentation Analysis
To learn more about this report, Download Free Sample
Paroxysmal Nocturnal Hemoglobinuria Therapeutics Market Insights, By Therapeutic Approach
Complement Inhibitors dominate the market share. This subsegment’s dominance stems from established efficacy and extensive clinical use, capturing over 60% of market revenue. Complement inhibitors’ targeted mechanism reduces hemolysis with a manageable safety profile, making them the frontline choice. Gene Therapy is the fastest-growing subsegment due to its potential to offer long-term remission by correcting genetic defects in hematopoietic stem cells. Its growth is propelled by promising clinical trial outcomes, increasing investor interest, and regulatory incentives, enhancing pipeline depth.
Paroxysmal Nocturnal Hemoglobinuria Therapeutics Market Insights, By Application
Classical PNH commands the highest market share. This segment benefits from greater clinical recognition and established treatment protocols, accounting for nearly 55% of market revenue. Classical PNH’s severe clinical manifestations drive demand for advanced therapeutics, contributing significantly to market growth. The fastest-growing subsegment is Aplastic Anemia Associated PNH, driven by increasing recognition of the overlap syndrome and growing research highlighting tailored therapeutic needs in this population.
Paroxysmal Nocturnal Hemoglobinuria Therapeutics Market Insights, By Distribution Channel
Hospital Pharmacy dominates the segment, representing over 50% of total market revenue, attributed to the requirement for supervised administration of complex PNH therapeutics and inpatient care protocols. Hospitals provide essential monitoring for adverse events, reinforcing their central role. Online Pharmacy is the fastest-growing distribution channel, benefiting from the increasing digitization of healthcare and patient preference for home delivery solutions. Growth in online channels is enhanced by telemedicine adoption and improved cold chain logistics, ensuring drug integrity.
Paroxysmal Nocturnal Hemoglobinuria Therapeutics Market Trends
Recent market trends indicate a strategic shift toward advanced gene therapy solutions and combination regimens.
The successful completion of multiple Phase III trials in 2024 has propelled gene therapies to the forefront, with one therapy achieving a 75% five-year survival rate in clinical cohorts.
Digital integration for patient monitoring has enhanced real-world evidence generation, improving market acceptance and compliance rates.
Biosimilars entering the market are expected to expand access in price-sensitive regions but may also encourage innovation in next-generation molecules.
Paroxysmal Nocturnal Hemoglobinuria Therapeutics Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Paroxysmal Nocturnal Hemoglobinuria Therapeutics Market Analysis and Trends
In North America, the dominance in the Paroxysmal Nocturnal Hemoglobinuria Therapeutics market is driven by a well-established healthcare infrastructure, robust R&D investments, and favorable regulatory frameworks, accelerating product approvals. The region held nearly 45% of the market share in 2025, propelled by the US's strong payer systems and widespread adoption of breakthrough therapies. Companies like Alexion and Apellis, capitalizing on this landscape, have seen consistent revenue growth.
Asia Pacific Paroxysmal Nocturnal Hemoglobinuria Therapeutics Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth, with a CAGR exceeding 14% from 2025 to 2032. This surge is attributed to improving diagnostic infrastructure, government-led rare disease initiatives, and increasing local manufacturing collaborations that reduce costs. Emerging economies like China and India are key growth contributors, with recently approved therapies and an expanding patient base.
Paroxysmal Nocturnal Hemoglobinuria Therapeutics Market Outlook for Key Countries
USA Paroxysmal Nocturnal Hemoglobinuria Therapeutics Market Analysis and Trends
The USA’s market remains the largest single-national contributor due to its advanced healthcare ecosystem and early adoption of novel therapeutics. In 2024, the US accounted for approximately 38% of global market revenue, supported by efficient reimbursement policies and a strong pipeline of clinical trials. The presence of leading companies investing heavily in R&D and commercialization, such as Alexion Pharmaceuticals, further solidifies its pivotal role.
Japan Paroxysmal Nocturnal Hemoglobinuria Therapeutics Market Analysis and Trends
Japan's market growth is driven by its aging population and increasing government focus on rare diseases under initiatives such as the Orphan Drug Act. In 2025, Japan witnessed a 20% increase in market revenue compared to 2023, underscored by faster regulatory approvals and expanding hospital networks. Major local pharma companies are collaborating with international firms to introduce advanced PNH treatment options, bolstering market expansion.
Analyst Opinion
The escalating market revenue is primarily driven by the surge in disease diagnosis rates due to advancements in hematologic testing and increased awareness. In 2024, improved diagnostic protocols in developed markets led to a 22% rise in detected cases compared to 2023. This influx directly expands patient pools eligible for advanced therapeutic regimens, supporting sustainable market growth.
Pricing strategies for complement inhibitors have evolved with competitive pressure and payer negotiations, resulting in a 5% average price reduction in the US market in 2025, enhancing patient accessibility. This adjusted pricing has correlated with a 15% higher uptake in therapeutic prescriptions during the first half of 2025 compared to the previous year.
Demand-side indicators highlight that expanding off-label uses and combination therapies are catalyzing overall market share growth. Recent clinical studies demonstrated a 30% improvement in patient outcomes when PNH therapeutics were integrated with immunosuppressants in transplant-related cases, attracting wider clinical adoption.
Supply chain optimization and increased production capacity, especially for monoclonal antibodies, have reduced lead times by nearly 20% in 2024, mitigating market restraints due to supply shortages. This efficiency has contributed to a more consistent global distribution network, critical for emerging markets.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: |
USD 1.2 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 11.5% | 2032 Value Projection: |
USD 2.6 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Apellis Pharmaceuticals, Alexion Pharmaceuticals (AstraZeneca), Novartis AG, Roche Holding AG, Alnylam Pharmaceuticals, Sobi (Swedish Orphan Biovitrum), Sanofi, Biomarin Pharmaceutical Inc., Rigel Pharmaceuticals, Ikaria Holdings, Hemlibra (Roche), Ionis Pharmaceuticals. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Paroxysmal Nocturnal Hemoglobinuria Therapeutics Market Growth Factors
The market growth is predominantly fueled by a rise in diagnosed PNH cases supported by advanced hematology diagnostics that have improved accuracy and reduced misdiagnosis. Collaborations between biotech firms and academic institutions have accelerated innovation in gene therapy, which offers curative hope, further enticing investments. Another growth driver includes expanded patient access driven by government and private insurance reimbursement frameworks, enhancing affordability. Lastly, the increasing pipeline of next-generation complement inhibitors with improved efficacy and safety profiles in late-stage clinical trials drives optimism in the market’s long-term revenue trajectory.
Paroxysmal Nocturnal Hemoglobinuria Therapeutics Market Development
In October 2025, crovalimab (Piasky) was approved in Taiwan, expanding access to one of the most advanced therapies for PNH in the Asia–Pacific region. The Taiwanese authorization emphasized the drug’s recycling-antibody engineering, which allows for sustained efficacy using comparatively lower doses. With this approval, Taiwan joins other markets prioritizing long-acting, patient-friendly complement inhibitors to reduce transfusion dependence and improve long-term quality of life for PNH patients.
In April 2024, the U.S. FDA granted approval to AstraZeneca’s danicopan (Vodeya), a first-in-class oral factor-D inhibitor designed as an add-on therapy for patients with PNH who continue to experience extravascular hemolysis despite treatment with C5 inhibitors. The approval introduced a new mechanism of complement control that can be used alongside existing therapies, addressing a persistent unmet need and offering patients an important step toward more personalized and comprehensive PNH management.
Key Players
Leading Companies of the Market
Apellis Pharmaceuticals
Alexion Pharmaceuticals (AstraZeneca)
Novartis AG
Roche Holding AG
Alnylam Pharmaceuticals
Sobi (Swedish Orphan Biovitrum)
Sanofi
Biomarin Pharmaceutical Inc.
Rigel Pharmaceuticals
Ikaria Holdings
Hemlibra (Roche)
Ionis Pharmaceuticals
Several leading companies are increasingly focusing on strategic acquisitions and collaborative trials. For instance, AstraZeneca’s acquisition of Alexion Pharmaceuticals in 2024 has notably expanded its portfolio in complement inhibition therapies, resulting in a 12% increase in related market revenue. Additionally, Roche's expansion in gene therapy development, demonstrated by their 2025 pipeline advancements, has fortified their competitive advantage and accelerated penetration in emerging markets.
Paroxysmal Nocturnal Hemoglobinuria Therapeutics Market Future Outlook
Future progress in PNH therapeutics will center on next-generation complement inhibitors, small-molecule oral drugs, and gene therapy. Advances in RNA and CRISPR-based technologies hold potential for curative approaches targeting the genetic root of the disease. Market expansion will also benefit from increasing awareness, early screening, and the approval of cost-effective biosimilars. Companies are investing heavily in long-term data generation to demonstrate safety and durability, positioning novel therapies as standard-of-care replacements for transfusion dependence. The continued convergence of rare disease funding and precision hematology research will sustain innovation momentum.
Paroxysmal Nocturnal Hemoglobinuria Therapeutics Market Historical Analysis
The PNH therapeutics market has evolved from purely supportive care to highly targeted complement-inhibition therapies. Before the early 2000s, management primarily involved blood transfusions and immunosuppressants to control symptoms. The introduction of eculizumab, a monoclonal antibody that inhibits complement protein C5, marked a revolutionary advancement by preventing intravascular hemolysis and reducing thrombosis risk. Subsequent innovations, including long-acting formulations like ravulizumab, improved dosing convenience and patient compliance, transforming disease prognosis and life expectancy.
Sources
Primary Research Interviews:
Hematologists
Immunologists
Biotech Researchers
Clinical Trial Investigators
Databases:
ClinicalTrials.gov
NIH Rare Disease Database
WHO Hematology Statistics
GlobalData Rare Disease Reports
Magazines:
Rare Disease Report
Hematology Times
Medical News Today
Nature Reviews Drug Discovery
Journals:
Blood Journal
The Lancet Hematology
Haematologica
Journal of Clinical Immunology
Newspapers:
The Guardian (Health)
The Wall Street Journal (Science)
The Hindu (Medical)
The Economic Times (Biotech)
Associations:
National Organization for Rare Disorders (NORD)
World Health Organization (WHO)
American Society of Hematology (ASH)
European Hematology Association (EHA)
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients