Parkinsons Disease Therapeutics Market is estimated to be valued at USD 5.85 Bn in 2025 and is expected to reach USD 9.39 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 7.0% from 2025 to 2032.
Analysts’ Views on Global Parkinsons Disease Therapeutics Market:
Parkinson’s disease is a brain disorder that mostly causes unintended or uncontrollable movements, such as shaking, stiffness, and difficulty with balance and coordination. Although there is no cure for the disease, market players are indulged in the production of innovative drugs that may reduce the symptoms of the Parkinsons disease therapeutics market. This is expected to increase the growth of the global Parkinsons disease therapeutics market, over the forecast period.
Figure 1. Global Parkinsons Disease Therapeutics Market Share (%), By Drug Class, 2025
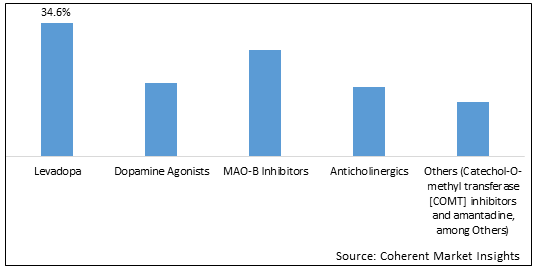
To learn more about this report, Download Free Sample
Global Parkinsons Disease Therapeutics Market– Drivers
Figure 2. Global Parkinsons Disease Therapeutics Market Share (%), By Region, 2025
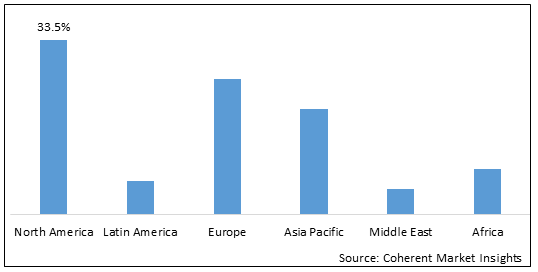
To learn more about this report, Download Free Sample
Global Parkinsons Disease Therapeutics Market- Regional Analysis
Among regions, North America is estimated to hold a dominant position in the global Parkinsons disease therapeutics market over the forecast period. North America is estimated to hold 33.5% of the market share in 2025 The global Parkinsons disease therapeutics market is expected to witness significant growth in the coming years, driven by the increasing research and development activities for the production of parkinson’s disease drugs by market players in the region. For instance, in 2022, Denali Therapeutics Inc., a clinical-stage biopharmaceutical company, and Biogen Inc., a global biotechnology company, a uniquely different pharmaceutical company are conducting a clinical trial to evaluate the efficacy and safety of BIIB122 (DNL151), as compared to placebo in approximately 640 participants with early-stage Parkinson’s disease. Biogen announced the initiation of dosing among the participants. The study is in Phase II, and the study is estimated to be completed by August 2025.
Global Parkinsons Disease Therapeutics Market– Impact of Coronavirus (COVID-19) Pandemic
The coronavirus (COVID-19) pandemic has had a devastating influence on the lives of many families across the world, as well as healthcare institutions and the global economy. During the COVID-19 epidemic, patients had trouble getting their regular Parkinson's disease therapeutics. For instance, in August 2020, according to the International Parkinson and Movement Disorder Society, the impact of COVID-19 on the quality of healthcare reported by Parkinson's disease patients found that COVID-19 reduced the quality of healthcare as well as worsened the symptoms and interrupted the access to medication for Parkinson’s disease patients. Access to PD medication was negatively impacted by closing the routine clinical space, faltering delivery systems, and the inability to access dispensaries or pay for medication.
Global Parkinsons Disease Therapeutics Market Segmentation:
The global Parkinsons disease therapeutics market report is segmented by drug class, by route of adminsitration, by distribution channel, and by region.
Parkinsons Disease Therapeutics Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 5.85 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.0% | 2032 Value Projection: | USD 9.39 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Teva Pharmaceutical Industries Ltd., Novartis AG, GlaxoSmithKline Plc., AbbVie Inc., Merck & Co., Inc., Zydus Cadila, Dr. Reddy’s Laboratories, Sun Pharmaceutical Industries Ltd., Cipla Inc., Boehringer Ingelheim International GmbH, Denali Therapeutics Inc., Biogen Inc., Prevail Therapeutics, Eli Lilly and Company and Voyager Therapeutics. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Parkinsons Disease Therapeutics Market- Cross Sectional Analysis:
Key players are producing drugs specific for the treatment of Parkinsons disease with advanced technology in the emerging economies and this is expected to boost demand Parkinsons disease therapeutics market in North America region. For instance, in May 2021, Zynex Inc., the manufacturer and marketer of medical devices headquatered in U.S., launched NeuroMove, neurological re-learning tool that promotes neuroplasticity & teaches healthy parts of the brain how to take over lost functionality. MeuroMove is a U.S. Food and Drug Administration-cleared for stroke rehabilitation, and its use has dramatically ameliorated functional impairments in stroke survivors in many clinical studies and in clinical practices.
Global Parkinsons Disease Therapeutics Market: Key Developments
Global Parkinsons Disease Therapeutics Market: Key Trends
Global Parkinsons Disease Therapeutics Market: Restraint
Global Parkinsons Disease Therapeutics Market - Key Players
Major players operating in the global Parkinsons disease therapeutics market include Teva Pharmaceutical Industries Ltd., Novartis AG, GlaxoSmithKline Plc., AbbVie Inc., Merck & Co., Inc., Zydus Cadila, Dr. Reddy’s Laboratories, Sun Pharmaceutical Industries Ltd., Cipla Inc., Boehringer Ingelheim International GmbH, Denali Therapeutics Inc., Biogen Inc., Prevail Therapeutics, Eli Lilly and Company and Voyager Therapeutics.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients