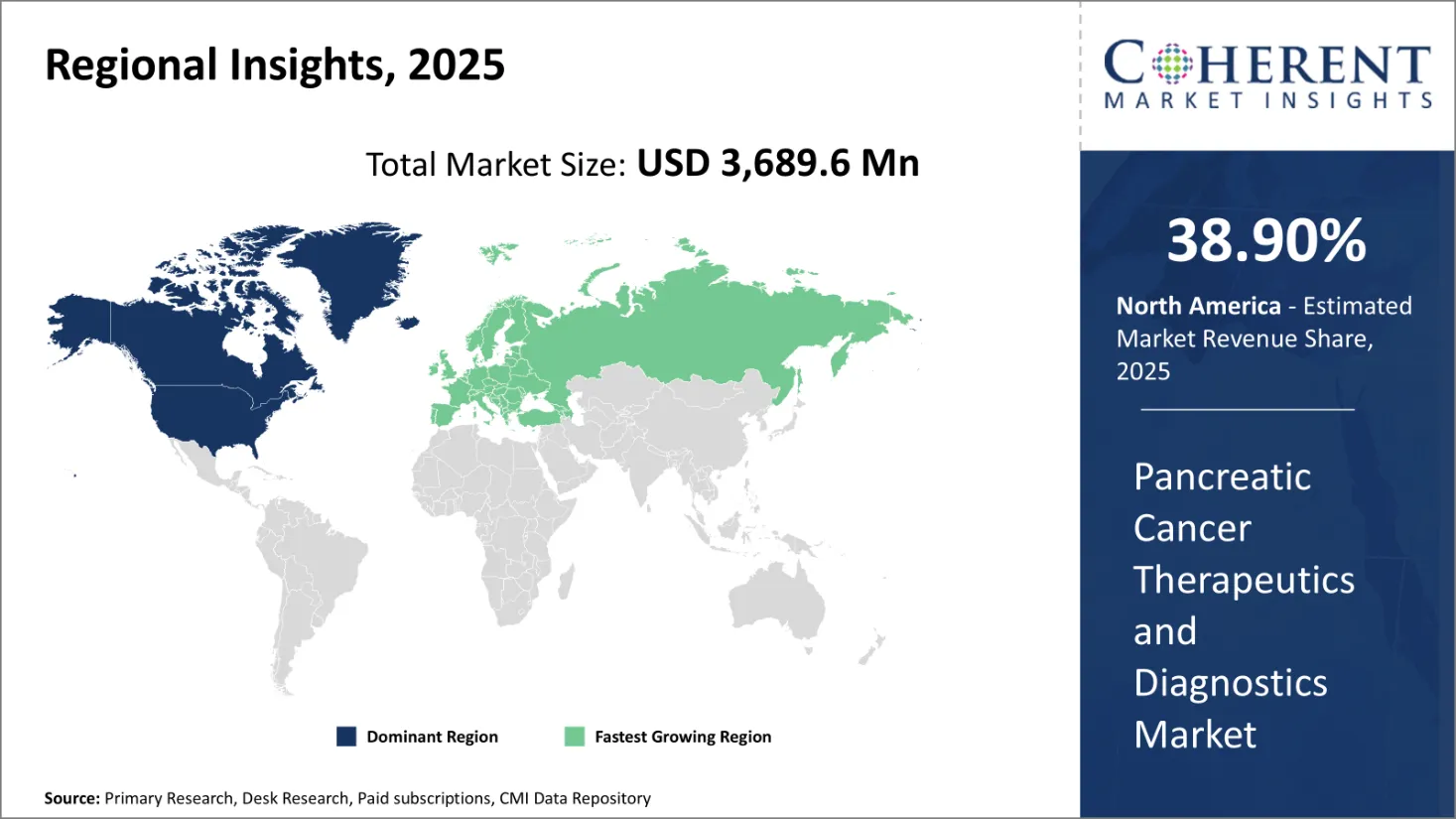
The Global Pancreatic Cancer Therapeutics and Diagnostic Market size was valued at USD 3,689.6 Mn in 2025 and is forecast to reach a value of USD 6,039.3 Mn by 2032 at a (CAGR) of 7.3% between 2025 and 2032.
The global pancreatic cancer therapeutics and diagnostic market forecast is experiencing strong growth due to the rise in incidence and prevalence of pancreatic cancer and rise in development of novel therapeutics. Moreover, growing geriatric population around the world and rise in demand for safe and effective therapeutics is expected to boost the growth of the market. However, factors such as stringent rules and regulations and high cost associated with diagnosis and treatments are expected to hamper the growth of the market.
|
Event |
Description and Impact |
|
Major Regulatory Approvals and Clinical Trial Advancements |
|
|
Diagnostic Technology Breakthroughs |
|
|
Strategic Industry Collaborations |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The pancreatic cancer therapeutics and diagnostics pipeline is robust, featuring several promising candidates across various clinical trial phases. Key molecules under development by leading innovators are outlined below:
This pipeline snapshot reflects dynamic progress in both therapeutic and diagnostic innovation aimed at improving early detection, personalized treatment, and patient outcomes in pancreatic cancer. The landscape continues to evolve as these candidates advance through clinical and regulatory milestones.
The therapeutics and diagnostics market for pancreatic cancer showcases vigorous innovation from competition which is indicative of a very strong patent prospect. Areas of active patent applications include:
Pharmaceutical, biotech, and diagnostic companies fiercely compete with one another as major patent holders like Roche, Novartis, Amgen, Illumina and Thermo Fisher Scientific emerges. Ostensibly, the rise in patent applications over the past three years demonstrates the sector's focus on self-driven technology advancement and high attention strategically geared towards personalized medicine.
Roughly speaking, the patent landscape is indicative of a strong clinical management innovation pipeline that is likely to alter pancreatic cancer treatment and profoundly shift the market.
The reimbursement framework of pancreatic cancer therapeutics and diagnostics incorporates critical coding constituents like ICD-10-CM (C25 for pancreatic cancer), CPT (e.g., 48150 for pancreatectomy), and HCPCS (e.g. J9999 for antineoplastic agents). Such codes assist in billing and claims processing within the healthcare systems.
These U.S. reimbursement rates are set by the Centers for Medicare & Medicaid Services (CMS), with Medicare entitled to reimburse hospital admissions, medical care, and FDA-authorized chemotherapy. In 2020, Medicare was estimated to have spent around $2.5 billion on managing pancreatic cancer, generally covering 80% of the approved expenses post deductibles.
Private insurers in the U.S. reported spending approximating $3.8 billion in 2020, each offering different coverage based on their plan types which include PPOs, HMOs, and HDHPs. Coverage is typically provided between 60 to 90 % post deductibles and copayments.
NICE assesses cost efficacy and recommends coverage to the NHS in the UK. Grasping reimbursement codes, processes, and insurance variation is pivotal to improving patient access and aiding in the growth of the market and services available for pancreatic cancer care.
When prescribing diagnostic and therapeutic options for pancreatic cancer, clinicians often choose from treatment frameworks that are clinically effective and incorporate a patient access pathway so as to minimize out-of-pocket expenses for patients.
For stakeholders looking to assist prescribers and foster more proactive involvement in the management of pancreatic cancer, these approaches in reimbursement strategies supporting specific preferences outline their expectations.
In terms of type, the Treatment segment is expected to dominate the pancreatic cancer therapeutics and diagnostics market demand with 80.7% in 2025, driven by the rising global demand for safe and effective treatment options. Increasing awareness about novel therapies, coupled with advancements in targeted drugs and immunotherapies, is accelerating adoption across healthcare systems worldwide. Leading pharmaceutical companies are focusing on developing precision treatments that improve patient outcomes while minimizing adverse effects.
The Diagnostics segment is also poised for significant growth, propelled by the escalating burden of pancreatic cancer globally. Early and accurate diagnosis remains critical for improving survival rates, leading to increased utilization of advanced imaging technologies, molecular diagnostics, and biomarker tests. Innovations aimed at enhancing diagnostic accuracy and accessibility are expanding the scope of pancreatic cancer detection and monitoring, thereby strengthening this segment’s market share.
To learn more about this report, Download Free Sample
North America is projected to dominate the global pancreatic cancer therapeutics and diagnostics market growth with 38.9% share in 2025, driven by a combination of high disease prevalence, advanced oncology care infrastructure, and strong pharmaceutical R&D activity. The region continues to lead in the adoption of cutting-edge diagnostic imaging, biomarker-based screening, and precision oncology therapies. According to the American Cancer Society, over 62,000 new cases of pancreatic cancer were reported in the U.S. in 2022, with nearly 50,000 deaths—highlighting a critical need for timely diagnosis and effective treatment strategies.
Healthcare institutions in North America benefit from robust clinical trial networks, favorable reimbursement policies, and significant public funding for cancer research. Ongoing collaboration between regulatory authorities, academic research centers, and private manufacturers supports rapid innovation and improved patient outcomes.
Europe is expected to hold the second-largest share of the global pancreatic cancer therapeutics and diagnostics market trend in 2025, underpinned by a rising disease burden, progressive public health strategies, and growing demand for precision medicine. According to United European Gastroenterology, pancreatic cancer now causes over 90,000 deaths annually across the EU, with mortality rates nearly doubling over the past 30 years.
The region benefits from structured cancer care pathways, expanding access to diagnostic innovations, and coordinated research funding at both national and EU levels. Increasing integration of molecular diagnostics and personalized treatment plans is strengthening Europe’s clinical response to pancreatic cancer.
Asia Pacific is anticipated to be the fastest-growing region in the global pancreatic cancer therapeutics and diagnostics market through 2025. The region’s growth is driven by increasing cancer incidence, rapid improvements in healthcare accessibility, and rising awareness around early detection. Countries across Asia Pacific are scaling up investments in oncology infrastructure, national screening programs, and data-driven cancer registries.
Efforts to enhance diagnostic coverage, combined with the presence of both domestic and multinational pharma companies, are fueling clinical advancements. Expansion of medical insurance schemes and public-private partnerships are also improving access to pancreatic cancer care.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 3,689.6 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.3% | 2032 Value Projection: | USD 6,039.3 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
F Hoffmann-La Roche AG, Merck KgaA, Apexigen Inc., Immunovia AB, Viatris Inc., Amgen Inc., AstraZeneca PLC, Bristol-Myers Squibb, Novartis AG, Pfizer Inc., Myriad Genetics Inc., Canon Medical Systems Corporation, FUJIFILM Holdings Corporation, Boston Scientific Corporation, and Rafael Holdings Inc. (Rafael Pharmaceuticals), among others. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
One of the key factors expected to augment the growth of the global pancreatic cancer therapeutics and diagnostic market over the forecast period is the rise in incidence and prevalence of pancreatic cancer around the world. According to the World Cancer Research Fund, pancreatic cancer is the 12th most common cancer across the globe, and it is the 12th most common cancer in men and the 11th most common cancer in women. Over 495,000 new cases of pancreatic cancer were diagnosed in 2020, increasing the demand for effective therapeutics and diagnostics.
Another factor which is driving the growth of the pancreatic cancer therapeutics and diagnostic market is the rise in development of novel therapeutics. Owing to increase in burden of pancreatic cancer worldwide and increase in awareness among people, players in the market are focusing on developing and launching novel therapeutics in the market.
In January 2022, Marker Therapeutics Inc. announced that the U.S. Food and Drug Administration (USFDA) Office of Orphan Products Development has granted Orphan Drug designation to MT-601, a multi-tumor-associated antigen-specific T cell therapy for the treatment of pancreatic cancer.
Growing geriatric population across the globe is expected to offer significant growth opportunities for players in the pancreatic cancer therapeutics and diagnostic market. For instance, the number of elderly patients with pancreatic cancer has increased worldwide, and thus, there is urgent need to develop specific therapeutics and diagnostic for elderly patients. According to the World Health Organization (WHO), by 2030, one in six people in the world will be aged 60 years and over, and by 2050, the world’s population of people aged 60 years and older will double (2.1 billion). This in turn is expected to drive growth of the market.
Increase in demand for safe and effective therapeutics worldwide is expected to offer significant growth opportunities for players in the pancreatic cancer therapeutics and diagnostic market. For instance, players in the market are focusing on developing and launching novel therapeutics and diagnostics in the market. In July 2021, Novartis announced that the U.S. FDA has granted Orphan Drug Designation (ODD) for NIS793 in combination with standard of care chemotherapy for the treatment of pancreatic cancer. NIS793 is known to have an important role in metastatic pancreatic ductal carcinoma (mPDAC) and other solid tumors.
With the increase in number of cancer cases and a steady rise in the geriatric population, demand for safe and effective treatment is also increasing. Thus, research institute and market players are increasingly investing for the development of effective therapeutics and diagnostics. In February 2021, Garvan Institute of Medical Research and the University of New South Wales Sydney led an innovative pancreatic cancer clinical trial program, thanks to a USD 3.75 million grant from the Cancer Institute NSW. This in turn is expected to drive the market growth.
Due to rise in burden of pancreatic cancer and increase in awareness among people, players in the market are focusing on launching and developing novel therapeutics. For instance, the U.S. Food and Drug Administration has approved several therapeutics to treat pancreatic cancer. In January 2022, the U.S. FDA gave orphan drug status to multi-targeted T-cell therapy for pancreatic cancer treatment. This in trend is also expected to continue over the forecast period.
The pancreatic cancer therapeutics and diagnostic market is highly competitive. This is attributed to the increase in demand for safe and effective therapeutics, as a result, players in the market are focusing on launching novel products in the market.
Some of the key players in the global pancreatic cancer therapeutics and diagnostic market are F Hoffmann-La Roche AG, Merck KgaA, Apexigen Inc., Immunovia AB, Viatris Inc., Amgen Inc., AstraZeneca PLC, Bristol-Myers Squibb, Novartis AG, Pfizer Inc., Myriad Genetics Inc., Canon Medical Systems Corporation, FUJIFILM Holdings Corporation, Boston Scientific Corporation, and Rafael Holdings Inc. (Rafael Pharmaceuticals), among others.
*Definition: Pancreatic cancer is cancer that forms in the cells of the pancreas. Pancreatic cancer occurs when changes (mutations) in the pancreas cells lead them to multiply out of control. The cancer can be diagnosed with the help of ultrasound, computerized tomography scans, magnetic resonance imaging and, sometimes, positron emission tomography scans. Treatment may include surgery, radiation, chemotherapy, or a combination of these.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients