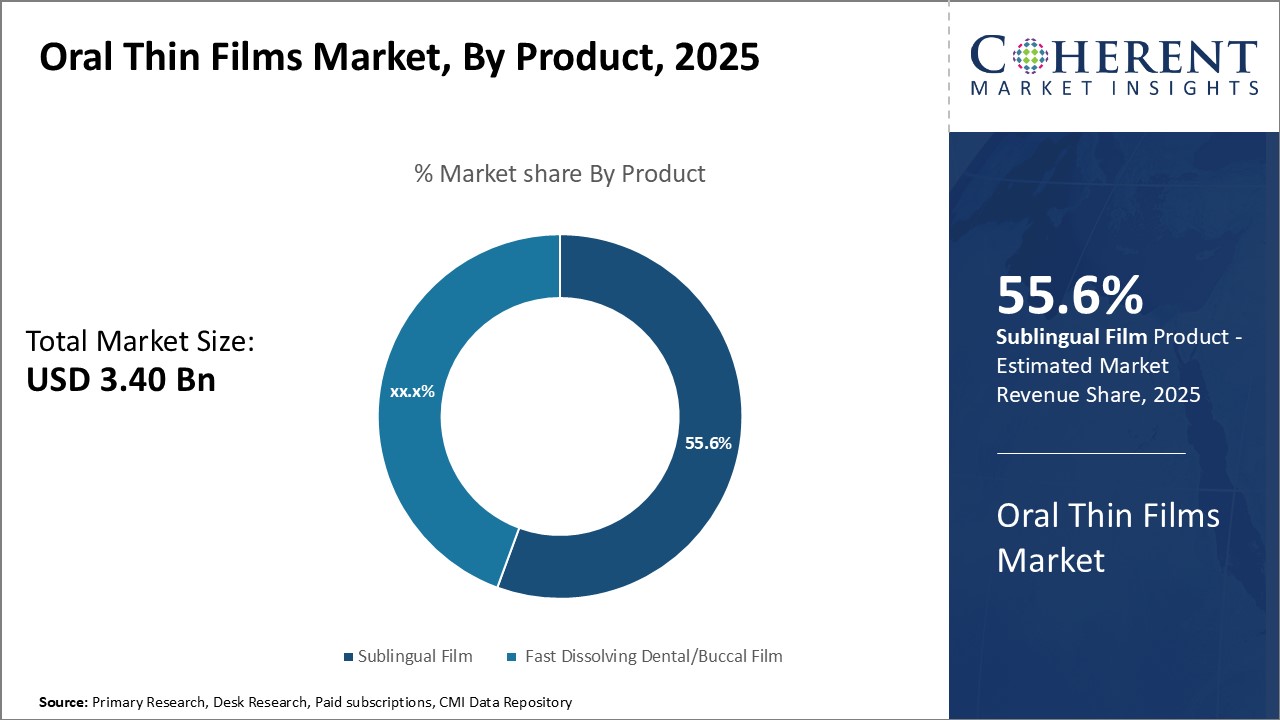
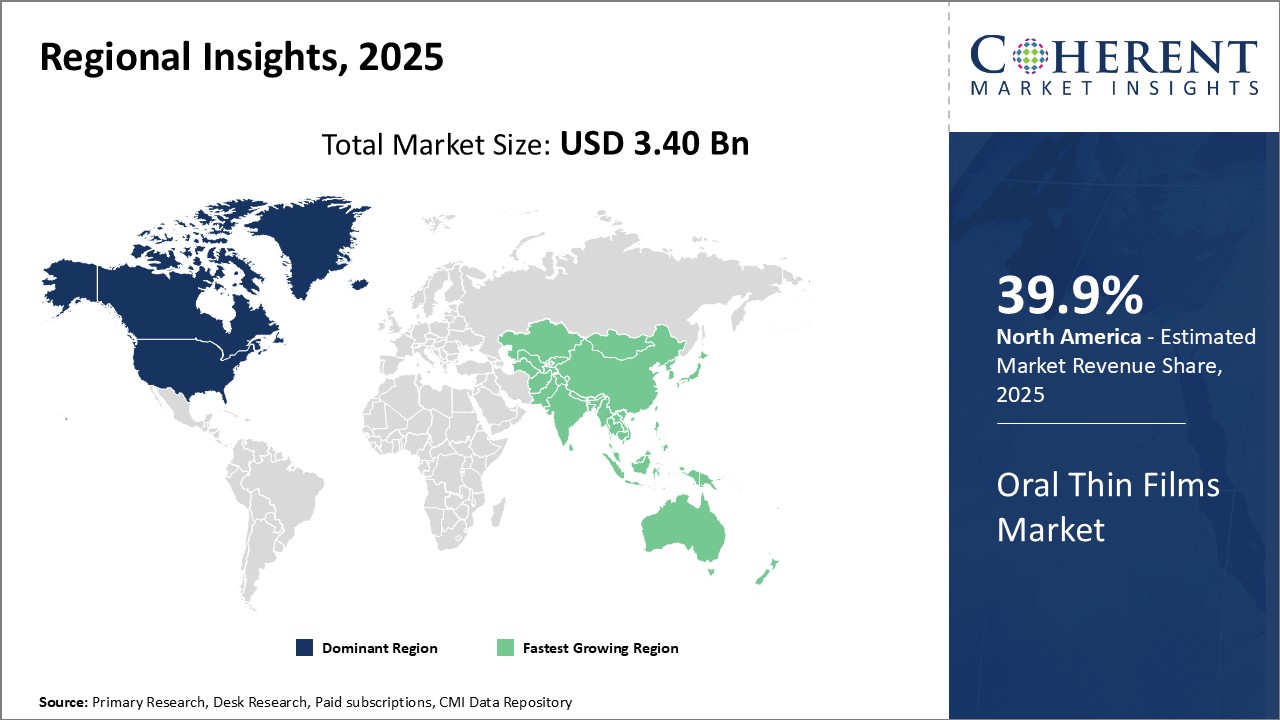
Global oral thin films market is estimated to be valued at USD 3.40 Bn in 2025 and is expected to reach USD 6.46 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 9.6% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
Oral thin film drug delivery technology offers advantages over conventional tablets and liquid formulations. Oral thin films are convenient to administer and cause less irritation to the oral cavity as compared to tablets. Rising preference for convenient dosages and technological advancements in thin film drug delivery are expected to drive the market growth during the forecast period. The younger generation populace is inclined toward convenient dosages which is further expected to boost the market growth. pipeline products in clinical trials and new product launches are anticipated to fuel the oral thin films market growth over the forecast timeframe. In January 2022, according to an article published by IntechOpen Oral films composed of composite polymeric matrices serve as effective drug delivery platforms. These matrices, comprising various components, often prioritize hydrophilic polymers. Both the European Medicines Agency (EMA) and the Food and Drug Administration (FDA) define a thin film that quickly dissolves in the oral cavity as an orodispersible film. Initially designed as breath-freshening formulations, oral films rapidly evolved to meet diverse commercial demands, offering a convenient and easily ingestible method for medication delivery.
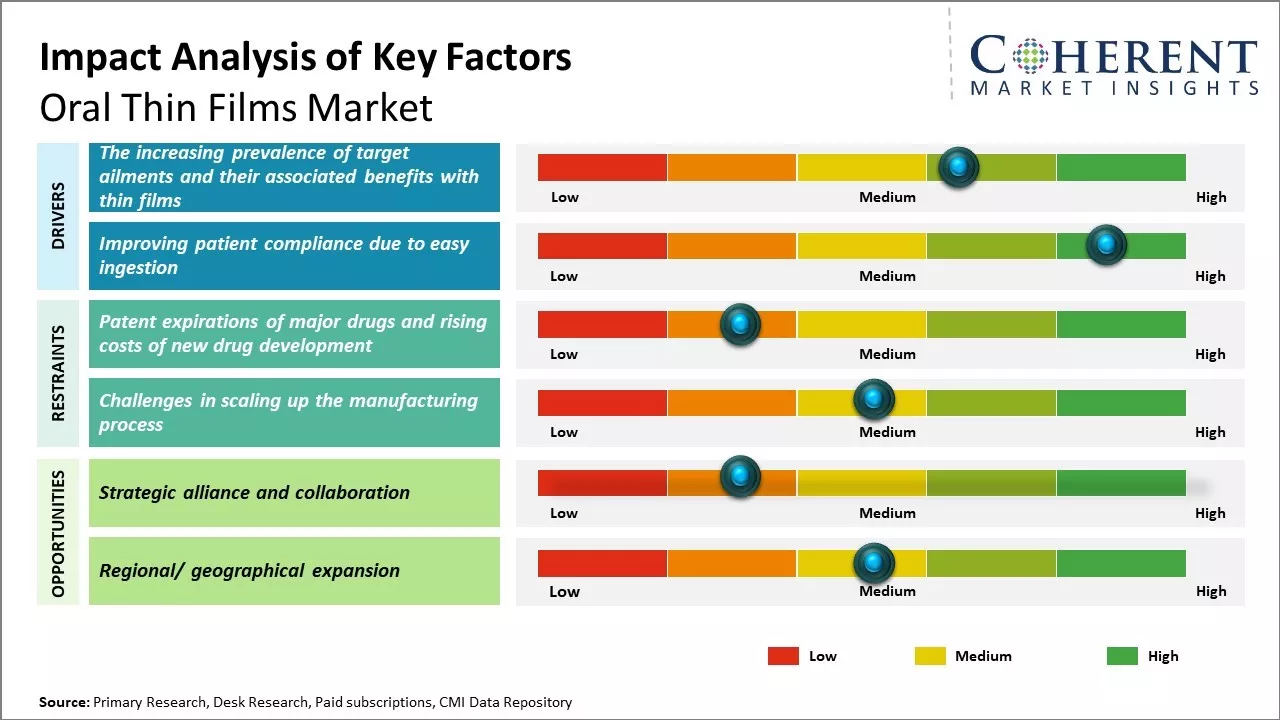
The increasing prevalence of target ailments and their associated benefits with thin films
The main factors propelling the market are the rising prevalence of target diseases and the benefits of thin films. For instance, according to data provided by Parkinson's Foundation in 2021 reported that over 10.0 million individuals worldwide were affected by Parkinson's disease. According to the same report, approximately 1.0 million Americans had Parkinson's disease in 2021. Furthermore, since Parkinson's disease risk rises with age, it is anticipated that the world's aging population will have a favorable effect on the market. In addition, the 2021 Parkinson's Foundation report projects that the number of individuals with Parkinson's disease will rise to 1.2 million by 2030. One of the most successful therapies for Parkinson's disease is thought to be oral thins. As a result, it is projected that rising rates of Parkinson's disease will fuel the demand for oral thins and accelerate market expansion.
Get actionable strategies to beat competition: Download Free Sample
Improving patient compliance due to easy ingestion
The convenience of oral thin films, which dissolve quickly without water, improves patient compliance by addressing swallowing difficulties and offering easy ingestion. This benefits pediatric, geriatric, and other patients who struggle with traditional oral dosage forms. Additionally, the ability to take oral thin films without water enhances medication adherence, especially for patients with limited water access or experiencing nausea. In hospitals, oral thin films simplify administration, contributing to their market growth. For instance, according to an article published by Research gate in February 2021, oral fast-dissolving films offer enhanced patient compliance, rapid release, and improved bioavailability, serving as a cost-effective alternative to conventional dosage forms. They facilitate the delivery of potent medications, extending patent life in the pharmaceutical industry. Recent research supports their industrialization. While offering convenience, their true potential will be realized with more available formulations.
Key Takeaways of Analyst:
The global oral thin films market is expected to witness steady growth over the forecast period driven by the increasing patient adherence to thin film products over traditional tablets and capsules. North America currently dominates the market owing to growing preference for convenient dosing formats. However, Asia Pacific is likely to emerge as the fastest growing region with rising healthcare expenditure and patient awareness in countries like China and India.
Major factors fueling market growth include the rising prevalence of various CNS Central nervous system and respiratory disorders among geriatric population globally which has triggered demand for easy-to administer drugs. Thin films also help address issues like dysphagia and provide quick action which is prompting companies to develop new products for conditions like nausea, migraine, etc. At the same time, regulatory approval process for oral thin films remains lengthy in some regions which can impede product launch plans of companies.
In terms of technology, organoleptic and zydis thin films have led the market so far but newer ones like porosystems and polymer films are gaining the attention of both drug makers and consumers owing to features like controlled drug release and longer shelf-life. However, limitation in drug solubility for certain molecules continues to challenge film formulators. Leading pharmaceutical companies as well as several start-ups have strengthened their thin film product portfolios through patents and acquisitions.
Market Challenges: Patent expirations of major drugs and rising costs of new drug development
The global oral thin films market is facing various challenges such as patent expirations of major drugs, surge in generics competition and rising costs of new drug development. Additionally, poor drug absorption of oral thin films for lipid-based formulations and lack of awareness among consumers about these films have hindered the market growth. High prices of thin film manufacturing equipment and R&D costs associated with drug delivery through oral thin films pose significant challenges for new players in this market. In February 2020, according to an article by contract pharma, the oral thin films (OTF) market faces challenges from expiring patents on major drugs and high costs of new drug development. Reformulating drugs for OTF delivery are becoming harder with more patents expiring. Developing new OTF formulations is costly, compounded by stringent regulations and patent expirations. Dr. Shanmugam emphasizes the need for specialized expertise and a targeted approach to OTF development to overcome these hurdles. Innovative strategies and robust R&D are crucial for sustaining the oral thin film market growth.
Market Opportunities: Strategic alliance and collaboration opportunities
Strategic actions like alliances and agreements amongst industry participants to broaden their presence in various geographic areas also contribute to the segment's growth. For instance, Sunovion Pharmaceuticals Inc., global biopharmaceutical company and BIAL, a pharmaceutical business, signed a deal in September 2021. According to the terms of the deal, Sunovion Pharmaceuticals Inc. gave BIAL the sole right to commercially license the apomorphine sublingual film (APL-130277) in Europe for the acute, intermittent treatment of episodes in people with Parkinson's disease (PD), it is approved under the brand name KYNMOBI.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
Insights, By Product Ease of administration increases preference for sublingual films
In terms of product, sublingual film contributes the highest share of the market 55.6% owing to its simplicity and convenience of administration compared to other oral thin film products. Sublingual films are small, thin sheets, or patches placed under the tongue for rapid absorption into the bloodstream through sublingual veins. This eliminates the first-pass metabolism that occurs when medication is swallowed and absorbed through the gastrointestinal tract, allowing for a faster onset of action than orally ingested tablets or liquids. The sublingual route also improves bioavailability for some active ingredients that may otherwise be broken down in the acidic environment of the stomach. The properties of sublingual films make them especially suitable for medications intended for acute or emergency use, such as treatments for migraine attacks, epileptic seizures, or opioid overdoses. Additionally, patients find sublingual films discreet and easy to carry as a portable dosage form. This wider acceptability and versatility has driven the greater demand and market adoption of sublingual films compared to alternative thin film products.
Insights, By Disease Indication: Greater awareness increases diagnosis and treatment rates for schizophrenia
In terms of disease indication, schizophrenia contributes the highest share of the market 55.6% due to increasing recognition of mental health issues. Schizophrenia is a complex and serious mental disorder affecting how a person thinks, feels, and behaves. It was long stigmatized and misunderstood, resulting in underdiagnosis and undertreatment. However, today schizophrenia is more widely known to be a common, biologically-based brain condition that often requires long-term medication management. Improved education around schizophrenia as a serious medical illness has encouraged more individuals experiencing symptoms to seek evaluation and care. Healthcare professionals are also better trained to recognize the signs and diagnose schizophrenia. Advanced antipsychotic medications formulated as oral thin films improve medication adherence for schizophrenia patients. This has contributed to higher treatment rates, driving significant demand within this disease segment of the global oral thin film market.
Need a Different Region or Segment? Download Free Sample
North America 39.9% has established itself as the dominant region in the global oral thin films market. The major factor contributing to its leadership position is the presence of several large pharmaceutical companies with strong capabilities for research and development of oral thin films. These companies are continuously investing in developing advanced oral thin film technologies and innovative formulations for both prescription and OTC medications. Furthermore, North America has a highly developed healthcare infrastructure and growing patient acceptance for innovative drug delivery formats like oral thin films.
The other key factor for North America's dominance is its substantial manufacturing capabilities for oral thin films. Many leading manufacturers have set up large scale, high output thin film manufacturing facilities in countries like the U.S. and Canada to cater to both domestic and international markets. This ensures the easy availability of a wide variety of oral thin films at competitive prices throughout the region. Strong intellectual property laws also encourage continuous innovation and new product development.
On the other hand, the Asia Pacific region is emerging as the fastest growing market for oral thin films. The major driving force behind its rapid growth is the expansions being carried out by multiple North American and European pharmaceutical companies. A growing number of large pharmaceutical firms have established modern oral thin film manufacturing units in countries like India and China to cater to the burgeoning demand. Moreover, several local pharmaceutical companies from emerging nations like India, China, and South Korea are also actively engaged in developing oral thin films for various therapeutic applications.
Asia Pacific also offers attractive growth opportunities owing to its huge patient population, rising disposable incomes, and increasing healthcare spending. Rapid urbanization and growing patient awareness about innovative drug delivery systems are further supporting the market growth. Abundant availability of raw materials, lower manufacturing costs, and a favorable regulatory environment makes Asia Pacific a highly lucrative production base for oral thin films. With continued investments and technology transfers from global leaders, Asia Pacific is well positioned to dominate the future growth of this market.
Oral Thin Films Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 3.40 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.6% | 2032 Value Projection: | USD 6.46 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
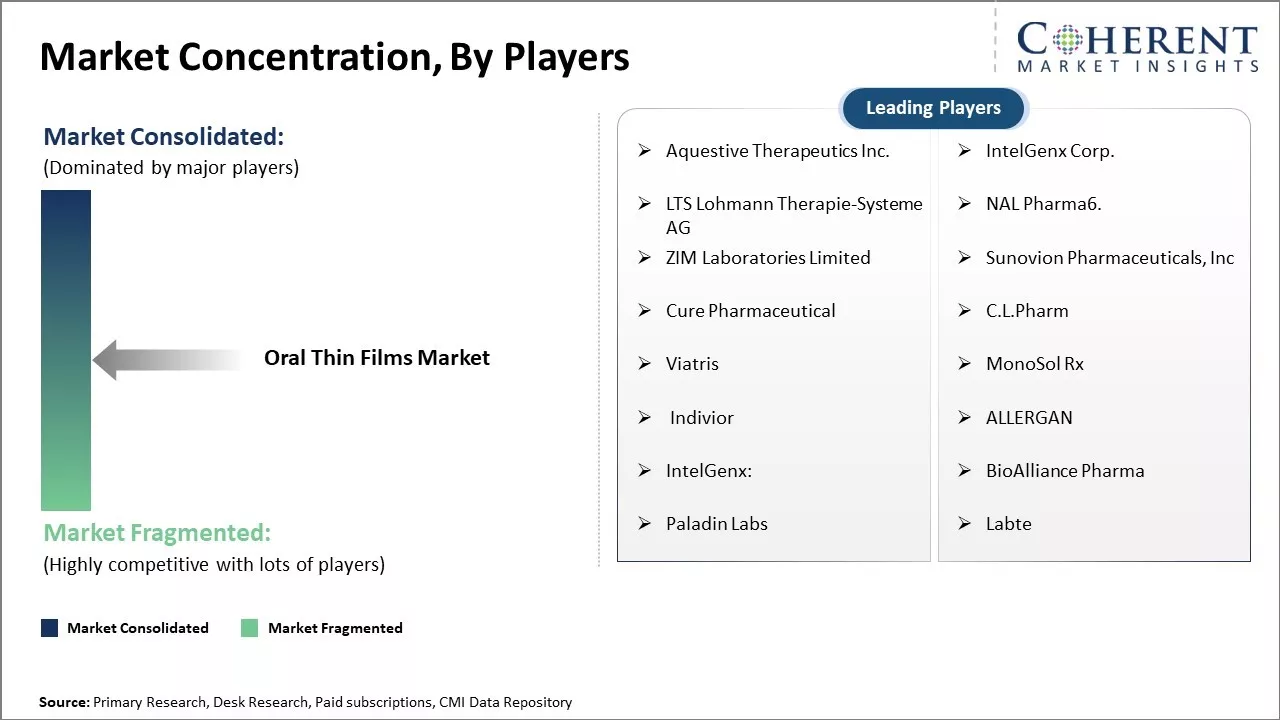
Aquestive Therapeutics Inc., IntelGenx Corp., LTS Lohmann Therapie-Systeme AG, NAL Pharma6., ZIM Laboratories Limited, Sunovion Pharmaceuticals, Inc, Cure Pharmaceutical, C.L.Pharm , Viatris, MonoSol Rx, Indivior, ALLERGAN, IntelGenx:, BioAlliance Pharma, Paladin Labs, and Labte |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients