Optical Coherence Tomography Devices Market Size and Forecast – 2025 – 2032
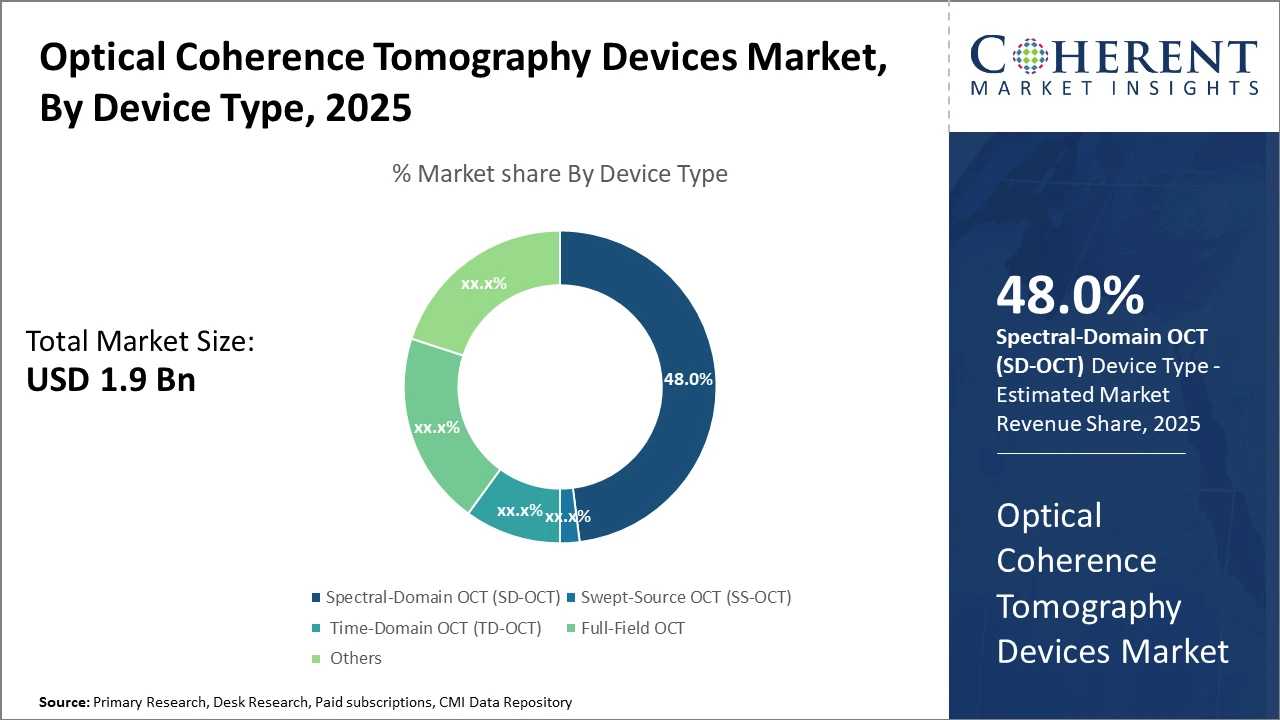
The Global Optical Coherence Tomography Devices Market size is estimated to be valued at USD 1.9 billion in 2025 and is expected to reach USD 3.4 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 8.2% from 2025 to 2032.
Global Optical Coherence Tomography Devices Market Overview
Optical coherence tomography devices are advanced, non-invasive imaging systems used to capture high-resolution cross-sectional images of biological tissues. These devices utilize low-coherence light to generate detailed, real-time images of retinal layers, coronary arteries, and other microstructures. OCT systems are widely used in ophthalmology for diagnosing retinal disorders, glaucoma, and macular degeneration, as well as in cardiology, dermatology, and oncology. Modern OCT devices offer enhanced imaging depth, faster scan speeds, and integration with digital health platforms, making them essential diagnostic tools in clinical practice.
Key Takeaways
Key takeaways based on segments highlight that the Spectral-Domain OCT subsegment holds the largest market share, driven by its superior image resolution that aligns with clinical demands for enhanced diagnostics.
Ambulatory Surgical Centers demonstrate rapid growth as adoption increases due to portability and shorter procedural times.
Ophthalmology remains the most dominant application, capturing a significant industry share fueled by chronic disease prevalence and preventive screening protocols.
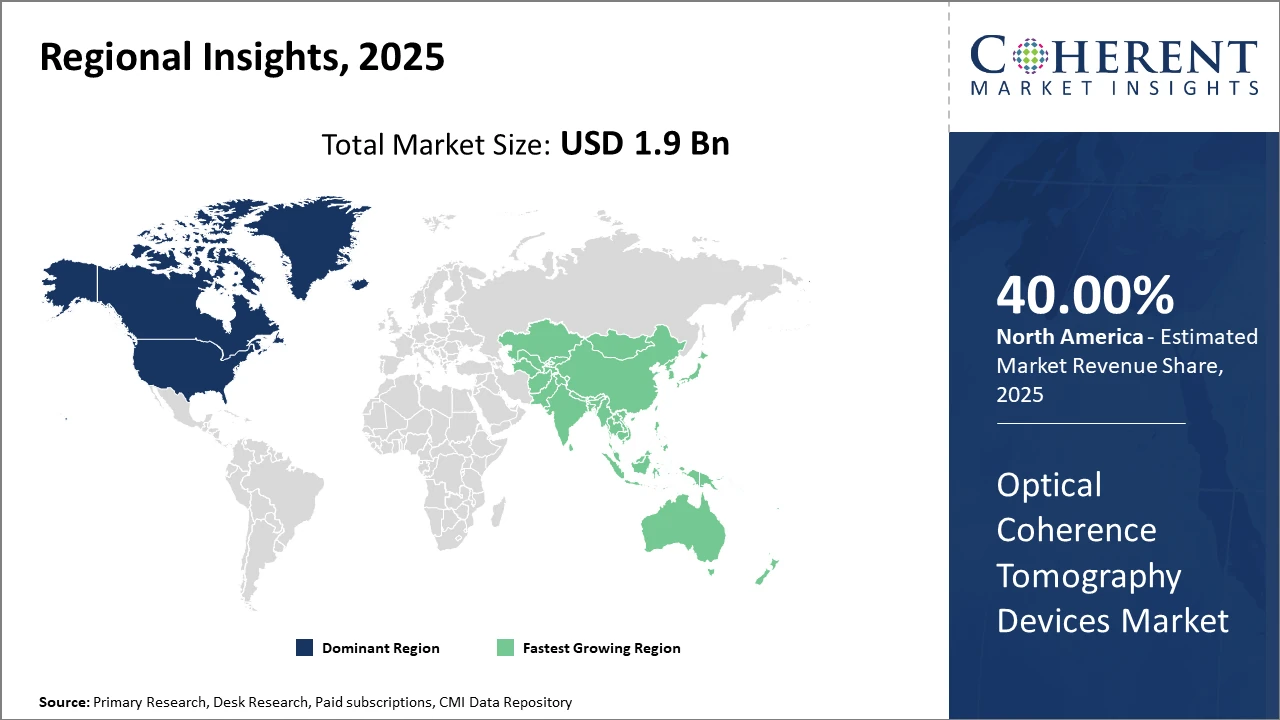
Regionally, North America maintains dominance with over 40% market share, attributable to strong healthcare infrastructure, early technology adoption, and favorable reimbursement strategies.
Meanwhile, Asia Pacific exhibits the fastest CAGR, supported by escalating healthcare investments, expanding clinical facilities, and growing awareness of eye health, particularly in China and India.
Optical Coherence Tomography Devices Market Segmentation Analysis
To learn more about this report, Download Free Sample
Optical Coherence Tomography Devices Market Insights, By Device Type
Spectral-Domain OCT dominates the market share with 48%. SD-OCT's dominance stems from its superior image resolution and faster scanning speed, which provide clinicians with enhanced diagnostic confidence, especially for retinal diseases. Swept-Source OCT, as the fastest growing subsegment, benefits from its deeper tissue penetration and improved visualization of choroidal structures, making it highly suitable for advanced ophthalmologic and cardiology applications. Time-Domain OCT, although largely supplanted in clinical applications, still retains a niche use in cost-sensitive environments.
Optical Coherence Tomography Devices Market Insights, By End-User
Hospitals & Clinics hold the largest share owing to their established patient base and broader investment capacity for high-end OCT systems. However, Ambulatory Surgical Centers represent the fastest-growing segment, fueled by the demand for portable and cost-efficient devices that enable rapid patient throughput. ASCs increasingly adopt handheld and compact OCT devices to perform preoperative and postoperative imaging. Diagnostic Centers continue to rely on specialized OCT for focused screening, while research institutes invest heavily in ultra-high-resolution devices for experimental development and clinical trials.
Optical Coherence Tomography Devices Market Insights, By Application
Ophthalmology undeniably dominates industry share due to OCT’s well-established role in managing retinal diseases, glaucoma, and diabetic retinopathy. Oncology is the fastest-growing application area, propelled by increasing utilization of OCT for imaging cancerous tissue microstructures and guiding surgical excisions, notably in dermatology and internal tumors. Cardiology employs OCT primarily in intravascular imaging to assess plaque morphology, marking steady growth. Dentistry and other emerging clinical fields remain small but show potential as technologies evolve and diagnostic benefits become clearer.
Optical Coherence Tomography Devices Market Trends
The Optical Coherence Tomography Devices market trend is strongly defined by the convergence of AI technologies and medical imaging.
In 2024, the launch of AI-augmented OCT devices by major market players enabled faster and more accurate diagnostics, reflecting a paradigm shift towards precision medicine.
Furthermore, there is a significant trend toward device portability; the introduction of handheld OCT devices over the past two years opened new frontiers in teleophthalmology and rural healthcare delivery.
Additionally, expanding OCT applications beyond ophthalmology—such as in cardiology for intravascular imaging—demonstrates the versatility that is driving market expansion and new revenue channels.
Optical Coherence Tomography Devices Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Optical Coherence Tomography Devices Market Analysis and Trends
In North America, the dominance in the Optical Coherence Tomography Devices market stems from robust healthcare infrastructure, advanced medical research institutions, and comprehensive reimbursement frameworks. The U.S., in particular, accounts for over 35% of total global industry share due to early adoption of cutting-edge imaging solutions and significant investments by leading market players such as Carl Zeiss Meditec AG and Topcon Corporation.
Asia Pacific Optical Coherence Tomography Devices Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth, with a CAGR surpassing 10%, fuelled by expanding healthcare infrastructure and rising government initiatives promoting eye care. China and India lead this growth, supported by increasing healthcare expenditure and rising patient awareness. The presence of key manufacturers investing in regional facilities and strategic distribution networks further accelerates market penetration across this territory.
Optical Coherence Tomography Devices Market Outlook for Key Countries
USA Optical Coherence Tomography Devices Market Analysis and Trends
The U.S. Optical Coherence Tomography Devices market benefits greatly from high healthcare spending and a technologically advanced medical device ecosystem. Regulatory approvals from agencies such as the FDA streamline the rollout of next-generation OCT devices. Leading firms like Heidelberg Engineering and Nidek continuously invest in R&D to cater to specialized ophthalmic and cardiologic applications. The surge in chronic eye conditions, coupled with telemedicine adoptio,n drives sustained demand.
China Optical Coherence Tomography Devices Market Analysis and Trends
China’s OCT device market shows rapid expansion propelled by government-backed healthcare reforms and increased hospital capacity upgrades. Domestic companies have begun collaborating with multinational firms to develop cost-effective models customized for local healthcare demands. The growing middle-class population and their higher health awareness levels contribute to faster acceptance of innovative imaging devices, positioning China as a key growth engine in the optical coherence tomography market trends.
Analyst Opinion
The rise in the geriatric population, particularly those aged 65 and above, correlates strongly with expanding market demand, as age-related macular degeneration and glaucoma prevalence continue to surge. In 2024, the U.S. Census Bureau reported a 3.1% increase in this demographic segment, underpinning the rising use of OCT devices across ophthalmology clinics. Early detection capabilities through OCT imaging serve as a critical driver in mitigating sight loss, enhancing the market size substantially.
Technological advancements such as swept-source OCT and enhanced depth imaging have significantly improved the resolution and penetration depth of ocular tissues. For instance, a 2025 clinical study published in the *Journal of Ophthalmic Research* demonstrated a 15% improvement in diagnostic precision using swept-source OCT compared to conventional models, fueling demand among premium clinical establishments and increasing average device pricing contributions to market revenue.
Expansion in emerging markets, especially in the Asia Pacific, is encouraged by growing healthcare infrastructure and rising healthcare expenditure. China’s Ministry of Health reported a 12% year-over-year increase in medical imaging investments in 2024, allowing hospitals to incorporate OCT devices into diagnostic centers more widely. This dynamic is a pivotal factor in extending the market horizon beyond mature regions.
The increasing integration of AI-based diagnostic algorithms within OCT devices redefines user experience and diagnostic output. A recent pilot in Japan involving a multi-institutional health system demonstrated 20% faster diagnostic turnaround and 30% improvement in disease classification accuracy by using AI-enhanced OCT systems. This trend is set to accelerate business growth and introduce new market dynamics oriented towards intelligent imaging solutions.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 1.9 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.2% | 2032 Value Projection: | USD 3.4 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Carl Zeiss Meditec AG, Topcon Corporation, Heidelberg Engineering, Nidek Co., Ltd., Optovue, Canon Inc., Santec Corporation, Leica Microsystems, Ellex Medical Lasers, LUMENIS. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Optical Coherence Tomography Devices Market Growth Factors
The Optical Coherence Tomography Devices market growth is deeply influenced by several critical drivers. First, the rising prevalence of chronic eye diseases worldwide actively drives demand, with glaucoma affecting over 80 million people globally according to the latest World Health Organization estimates in 2024. Second, the surge in healthcare expenditure, particularly in the Asia Pacific and North America, facilitates the acquisition of cutting-edge OCT systems in leading hospitals and diagnostic centers. Third, advancements in device technology, including higher speed and resolution, widen applicability beyond ophthalmology into oncology and cardiology diagnostics, broadening the market scope. Fourth, supportive policies and reimbursement frameworks in developed economies accelerate adoption rates, making OCT devices more accessible in clinical settings. These factors collectively stimulate steady market expansion and robust revenue generation.
Optical Coherence Tomography Devices Market Development
In 2024, NIDEK launched the RS-1 Glauvas Optical Coherence Tomography system, specifically designed for comprehensive glaucoma diagnosis and follow-up. The system combines high-resolution OCT imaging with advanced analytical tools for optic nerve head, retinal nerve fiber layer, and ganglion cell analysis, offering clinicians greater confidence in glaucoma detection and progression monitoring.
In 2024, Optovue introduced an AI-integrated OCT platform, leveraging artificial intelligence to improve image interpretation accuracy and streamline clinical workflows. The system supports faster detection of retinal abnormalities, helping clinicians make more consistent and data-driven diagnostic decisions.
Key Players
Leading Companies of the Market
Carl Zeiss Meditec AG
Topcon Corporation
Heidelberg Engineering
Nidek Co., Ltd.
Optovue
Canon Inc.
Santec Corporation
Leica Microsystems
Ellex Medical Lasers
LUMENIS
Competitive strategies among these market companies emphasize mergers and acquisitions, launch of AI-integrated OCT platforms, and strategic collaborations to expand geographic reach. For example, Carl Zeiss Meditec AG’s 2024 partnership with a leading AI imaging company augmented its device portfolio with predictive analytics capabilities, enhancing clinical workflow and boosting sales. Similarly, Topcon Corporation expanded its market access in Asia through joint ventures in China and India, increasing its market share substantially in 2024.Additionally, companies are channeling resources toward miniaturization of OCT devices, allowing portable and handheld devices to penetrate untapped end-use segments such as ASC and remote diagnostic centers, addressing emerging market challenges like accessibility and affordability.
Optical Coherence Tomography Devices Market Future Outlook
The future outlook for the OCT devices market is highly promising as demand for non-invasive, high-precision diagnostics continues to increase. Technological innovation is expected to focus on artificial intelligence–enabled image analysis, cloud-based data integration, and multi-modal imaging platforms. The expansion of OCT applications into cardiology, dermatology, and neurology will further broaden the market scope. Aging populations and rising prevalence of chronic eye diseases will sustain long-term demand. Additionally, the development of portable and cost-effective OCT systems is expected to improve access in outpatient clinics and emerging healthcare markets.
Optical Coherence Tomography Devices Market Historical Analysis
The optical coherence tomography devices market has evolved steadily since its introduction as a specialized imaging technology primarily used in research and tertiary care hospitals. In its early years, OCT adoption was limited due to high system costs, bulky equipment, and relatively slow image acquisition speeds. As healthcare providers increasingly emphasized early disease detection, technological advancements such as spectral-domain and swept-source OCT significantly improved resolution, scan depth, and speed. These improvements expanded the use of OCT beyond academic centers into routine ophthalmology clinics. Over time, OCT became an essential diagnostic tool for managing retinal disorders, glaucoma, and macular degeneration, establishing a strong foundation for market growth across developed healthcare systems.
Sources
Primary Research Interviews:
Ophthalmologists
Medical Imaging Specialists
Hospital Procurement Managers
Biomedical Engineers
Device Distributors
Databases:
Statista Medical Imaging
GlobalData MedTech
WHO Vision Data
FDA Medical Device Database
OECD Health Statistics
Magazines:
Ophthalmology Times
Medical Device Network
Diagnostic Imaging
MedTech Dive
Healthcare IT News
Journals:
Investigative Ophthalmology & Visual Science
Ophthalmology Journal
Biomedical Optics Express
Journal of Vision
Clinical Ophthalmology
Associations:
American Academy of Ophthalmology
International Society for Optics and Photonics
WHO
MedTech Europe
Vision Council
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients