Opioid Use Disorder Market is estimated to be valued at USD 3.83 Bn in 2025 and is expected to reach USD 6.87 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 8.7% from 2025 to 2032.
Analysts’ Views on Global Opioid Use Disorder Market:
Increasing investment & funding by government to combat the overdose epidemic of opioids is expected to drive growth of global opioid use disorder market over the forecast period. For instance, on September 23, 2022, the U.S. Department of Health and Human Services’ (HHS) Overdose Prevention Strategy, the Health Resources and Services Administration (HRSA), announced investment of over US$ 104 Mn to help clinicians who can prescribe the opioid treatment buprenorphine. Moreover, on August 21, 2022, the U.S. Department of Health and Human Services (HHS) through the Substance Abuse and Mental Health Services Administration (SAMHSA), announced the award of US$ 79.1 Mn in overdose prevention grants, as part of President Biden’s National Drug Control Strategy, the HHS Overdose Prevention Strategy, and the Biden-Harris Unity Agenda to address the opioid and overdose epidemic.
Figure 1. Global Opioid Use Disorder Market Share (%), By Drug Type, 2025
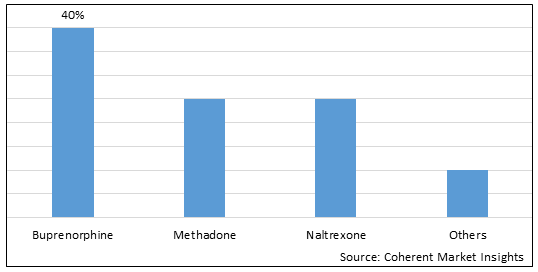
To learn more about this report, Download Free Sample
Global Opioid Use Disorder Market– Drivers
Rising prevalence of opioid use disorder
Rising prevalence of opioid use disorder among the population is expected to drive growth of the the global opioid use disorder market over the forecast period. For instance, in 2020, according to the data published by National Institute on Drug Abuse, an estimated 2.7 Mn people aged 12 or older, in the U.S. had an opioid use disorder (OUD) including 2.3 Mn people with a prescription opioid use disorder.
Increasing organic growth strategies such as product approval
Increasing organic growth strategies such as product approval by regulatory authorities to expand product portfolio of the key market players is expected to drive growth of the global opioid use disorder market over the forecast period. For instance, in April 2020, Amneal Pharmaceuticals, Inc., a pharmaceutical company, announced that it had received abbreviated new drug application (ANDA) approval from the U.S. Food and Drug Administration (FDA) for a generic version of butrans (buprenorphine) transdermal system, 5 mcg/hr, 7.5 mcg/hr, 10 mcg/hr, 15 mcg/hr and 20 mcg/hr. Amneal Pharmaceuticals, Inc. was granted the competitive generic Ttherapy (CGT) designation and 180 days of exclusivity for the 7.5 mcg/hr dose.
Figure 2. Global Opioid Use Disorder Market Share(%), By Region, 2025
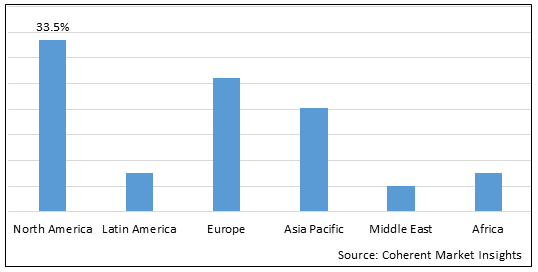
To learn more about this report, Download Free Sample
Global Opioid Use Disorder Market- Regional Analysis
Among region, North America is estimated to hold a dominant position in the global opioid use disorder market over the forecast period. North America is estimated to hold 33.5 % of the market share in 2025. The market is expected to witness significant growth in the near future, owing to increasing organic growth strategies such as product approval by regulatory authorities to expand product portfolio of the key market players. For instance, in December 2021, Camurus AB, a pharmaceutical company, announced that its U.S. licensee Braeburn had received a Complete Response Letter (CRL) from the U.S. Food and Drug Administration (FDA) for its updated New Drug Application (NDA) for Brixadi (buprenorphine) extended-release injections for the treatment of opioid use disorder. The CRL is a result of continued quality related deficiencies at Braeburn’s U.S. based third party manufacturer, identified by the FDA during a pre-approval inspection.
Opioid Use Disorder Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 3.83 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.7% | 2032 Value Projection: | USD 6.87 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Indivior PLC, Alkermes, Orexo AB, Titan Pharmaceuticals, Inc, Teva Pharmaceutical Industries Ltd., Mallinckrodt Pharmaceuticals, BioDelivery Sciences International Inc., Viatris Inc., Hikma Pharmaceuticals PLC, Camurus AB, Sun Pharmaceutical Industries Ltd., Amneal Pharmaceuticals LLC., Purdue Pharma LP, Alvogen, Faran Shimi Pharmaceutical, Novartis AG, Emplicure AB, MODASA Pharmaceuticals Pvt. Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Opioid Use Disorder Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease had spread to over 100 countries across the globe, and the World Health Organization (WHO) declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others faced problems regarding transportation of drugs from one place to another.
However, the COVID-19 had negative impact on the global opioid use disorder market, as patients with opioid use had limited access to opioid treatment programs during the COVID-19 public health emergency, whereas access to buprenorphine, an opioid drug treatment, remained undisrupted. For instance, on March 11, 2022, According to a report published by JAMA Health Forum, an international peer-reviewed journal (American Medical Association), patients with opioid use disorder (OUD) are less likely to receive opioid treatment due to disruptions in care during the COVID-19 pandemic. Access to the program has decreased. Opioid-related overdoses during the COVID-19 pandemic in the U.S. have decreased by 14% from 0.34 per 100,000 patients before the pandemic to 0.29 per 100,000 patients at the start of the pandemic. The recorded death rate is 69,710. 2020 is the 19th time a pandemic has occurred.
Global Opioid Use Disorder Market- Segmentation
Global opioid use disorder market is segmented into drug type, route of administration, distribution channel, and region.
Among all the segmentation, the drug type segment is expected to dominate the market over the forecast period due to increasing organic growth strategies such as product launch by key market players to expand their product portfolio. For instance, in May 2020, Hikma Pharmaceuticals PLC., the multinational generic pharmaceutical company, launched buprenorphine hydrochloride injection, 0.3mg/mL, the generic version of buprenex [1] in the U.S. through its U.S. affiliate, Hikma Pharmaceuticals U.S.A.Inc.
Global Opioid Use Disorder Market- Cross Sectional Analysis
In Drug type segment buprenorphine is dominant in Europe region due to increasing organic growth strategies such as product approvals by regulatory authorties to expand key market’s players product portfolio. For instance, in November 2021, Camrus AB, a biotechnology company, announced that the European Medicines Agency (EMA) had accepted the company’s submission of a Type II variation application for Buvidal (buprenorphine) prolonged release injection to include the treatment of chronic pain.
Global Opioid Use Disorder Market: Key Developments
Global Opioid Use Disorder Market: Trends
Global Opioid Use Disorder Market: Restraint
Global Opioid Use Disorder Market- Key Players
Major players operating in the global opioid use disorder market include Indivior PLC, Alkermes, Orexo AB, Titan Pharmaceuticals, Inc, Teva Pharmaceutical Industries Ltd., Mallinckrodt Pharmaceuticals, BioDelivery Sciences International Inc., Viatris Inc., Hikma Pharmaceuticals PLC, Camurus AB, Sun Pharmaceutical Industries Ltd., Amneal Pharmaceuticals LLC., Purdue Pharma LP, Alvogen, Faran Shimi Pharmaceutical, Novartis AG, Emplicure AB, MODASA Pharmaceuticals Pvt. Ltd.
Global Opioid Use Disorder Market : Definition: Opioid use disorder is a serious health issue that is rising globally in recent years. There are various medication-assisted treatment (MAT) options available to help patients who are suffering from this disorder. The main products used for the treatment are methadone, buprenorphine, and naltrexone. A deadly consequence of the opioid crisis is increased incidence of blood-borne infections, including hepatitis B virus and hepatitis C, human immunodeficiency virus (HIV), and bacteria that cause heart infections (endocarditis ).
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients