Opioid Induced Constipation Treatment Market Size and Forecast – 2026 – 2033
The Global Opioid Induced Constipation Treatment Market size is estimated to be valued at USD 1.62 billion in 2026 and is expected to reach USD 2.95 billion by 2033, exhibiting a compound annual growth rate (CAGR) of 8.9% from 2026 to 2033.
Global Opioid Induced Constipation Treatment Market Overview
The opioid-induced constipation (OIC) treatment market includes a range of products designed to relieve constipation caused by long-term opioid use. These products primarily consist of peripherally acting mu-opioid receptor antagonists (PAMORAs), which target opioid receptors in the gastrointestinal tract without affecting pain relief. Commonly used therapies also include laxatives, stool softeners, and prosecretory agents that improve bowel movement frequency and consistency. Growing opioid prescriptions, rising awareness of OIC, and advancements in gastrointestinal therapies continue to drive demand for these treatment products worldwide.
Key Takeaways
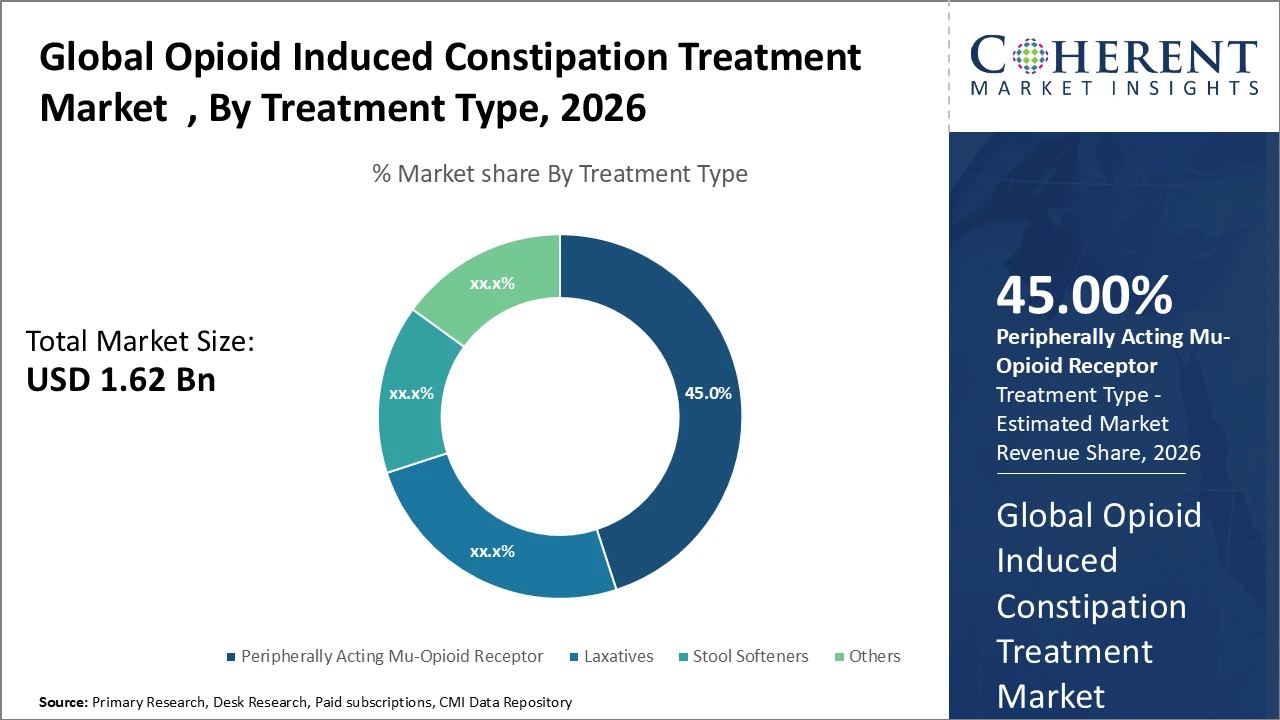
The PAMORA segment dominates the opioid induced constipation treatment market, accounting for 45% of the industry share, driven by high clinical efficacy and growing physician preference for targeted therapies.
Hospital pharmacies constitute the largest distribution channel, holding a 52% market share, supported by the strong involvement of healthcare institutions in the treatment and management of OIC patients.
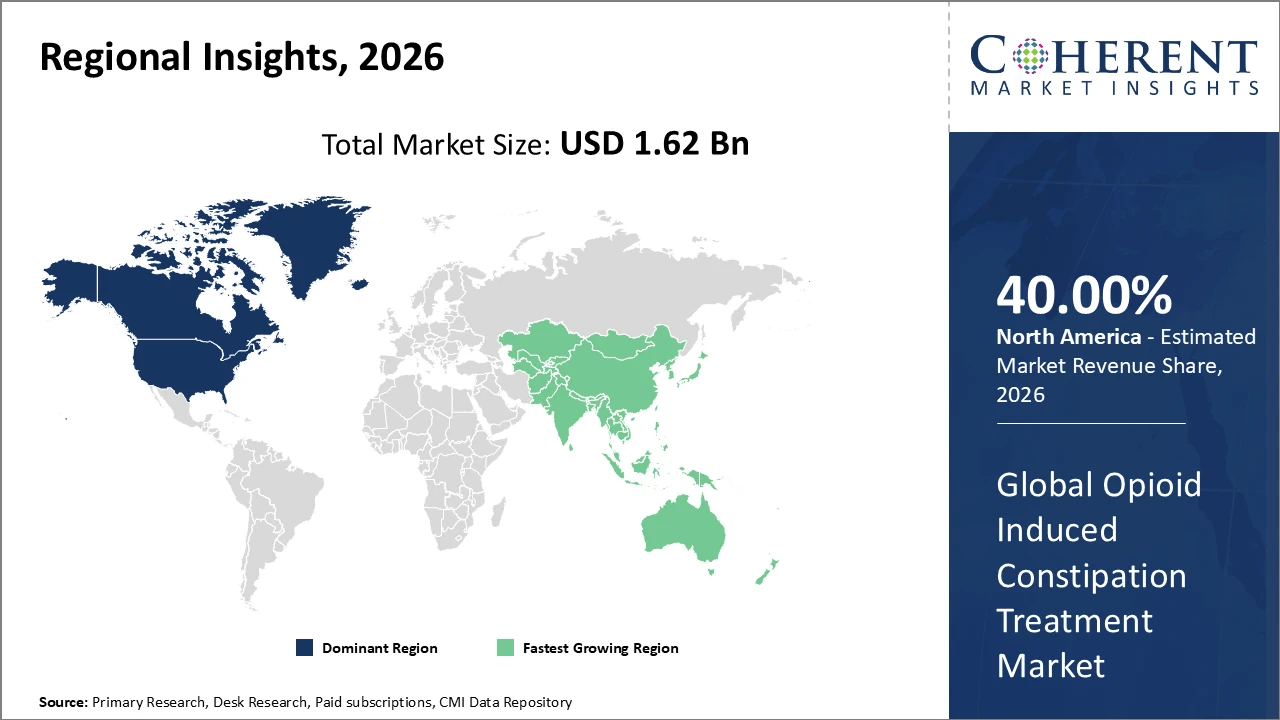
North America leads the market in terms of revenue, supported by high opioid prescription volumes, advanced healthcare infrastructure, and early adoption of innovative treatment options.
Asia Pacific is expected to witness the fastest growth, with a CAGR exceeding 9%, driven by a rapidly aging population, improving healthcare access, and rising expenditure on chronic disease management.
Opioid Induced Constipation Treatment Market Segmentation Analysis
To learn more about this report, Download Free Sample
Opioid Induced Constipation Treatment Market Insights, By Treatment Type
Peripherally acting mu-opioid receptor antagonists (PAMORAs) dominate the market with a 45% share, driven by their targeted mechanism that effectively reverses opioid-induced constipation without compromising analgesic effects. Their growing adoption is supported by strong clinical evidence demonstrating superior efficacy and a lower incidence of adverse events compared to conventional therapies, including 2024 clinical trials reporting a 25% improvement in patient quality of life. Laxatives continue to be widely used due to their affordability and easy availability; however, they are generally less effective in managing OIC and are primarily used as adjunct therapies. Stool softeners serve a limited role in mild cases and for maintenance therapy. The “others” segment comprises emerging probiotic- and fiber-based treatments, which are gaining gradual acceptance but currently account for a small share of the market.
Opioid Induced Constipation Treatment Market Insights, By Route of Administration
Oral administration dominates usage due to its ease of dosing and high patient compliance, accounting for nearly 60% of the market share. This dominance is further supported by innovations such as extended-release oral formulations introduced in late 2025, which offer prolonged symptom relief and improved convenience. Injectable routes are primarily utilized in acute care settings and among patients who cannot tolerate oral medications, making them the fastest-growing subsegment. Adoption of injectable therapies has increased notably in hospital pharmacies, supported by new product launches that recorded a 15% year-on-year growth during 2024–2025. Rectal administration remains restricted to specific clinical situations and maintains a stable but limited presence. The “others” category, which includes suppositories and emerging delivery technologies, is still under development and currently contributes minimally to overall market growth.
Opioid Induced Constipation Treatment Market Insights, By End-User
Hospital pharmacies dominate the distribution landscape with a 52% market share, reflecting their central role in managing opioid prescriptions and administering treatments for opioid-induced constipation. This dominance is supported by integrated healthcare systems and favorable reimbursement frameworks that facilitate access to advanced therapies. Retail pharmacies remain key access points for outpatient prescriptions and maintain a stable share, particularly in regions with well-established pharmacy networks. Online pharmacies represent the fastest-growing distribution channel, especially after 2024, driven by digital transformation and increased adoption of telemedicine, which enhances patient reach and convenience. The “others” segment includes specialized compounding pharmacies and emerging direct-to-consumer models, contributing modestly as healthcare delivery models continue to evolve.
Opioid Induced Constipation Treatment Market Trends
The opioid induced constipation treatment market is witnessing a strong shift toward biologics and receptor-targeted therapies, supported by increasing clinical evidence that positions PAMORAs as a superior treatment option.
In 2025, AstraZeneca’s launch of a novel PAMORA formulation recorded a 15% higher market uptake compared to earlier drugs in the same class, reflecting growing clinician and patient confidence.
Integration of digital adherence monitoring is emerging as a key trend, with platforms developed in North America improving therapy compliance by more than 12% in recent years.
Combination therapies that integrate analgesic and OIC treatments are gaining traction, as they simplify treatment regimens and enhance patient quality of life, supported by clinical studies published in 2024.
Opioid Induced Constipation Treatment Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Opioid Induced Constipation Treatment Market Analysis and Trends
In North America, dominance in the opioid induced constipation treatment market is driven by a well-established healthcare infrastructure, high opioid prescription volumes, and favorable reimbursement policies. The region accounts for over 40% of the market share in 2026, supported by the strong presence of leading pharmaceutical companies such as AstraZeneca and Pfizer with extensive commercial reach and innovative therapy portfolios. Supportive regulatory frameworks have enabled faster product approvals and launches, while strategic collaborations with hospital systems and healthcare providers have further strengthened market penetration across the region.
Asia Pacific Opioid Induced Constipation Treatment Market Analysis and Trends
Asia Pacific is experiencing the fastest growth in the opioid induced constipation treatment market, with a CAGR exceeding 9%, fueled by expanding healthcare infrastructure, a rising elderly population, and increasing opioid use for cancer and chronic pain management. Countries such as China and India are seeing rapid growth in healthcare expenditure, improving market access and awareness of OIC treatments. Both local companies and multinational players are capitalizing on these trends to launch more affordable and innovative therapies, further driving market expansion across the region.
Opioid Induced Constipation Treatment Market Outlook for Key Countries
USA Opioid Induced Constipation Treatment Market Analysis and Trends
The United States continues to lead the opioid induced constipation treatment market, driven by extensive opioid consumption, which reached around 46.7 prescriptions per 100 persons in 2024. Leading pharmaceutical companies are leveraging high healthcare spending, investing significantly in research and development of effective OIC therapies. AstraZeneca and Pfizer have reinforced their presence through new product launches and strategic hospital partnerships, contributing significantly to U.S. market revenue. The country’s focus on innovation and supportive regulatory frameworks encourages early adoption of advanced treatments, setting benchmarks that influence global market trends.
Germany Opioid Induced Constipation Treatment Market Analysis and Trends
The opioid-induced constipation treatment market in Germany is characterized by steady growth, driven by high opioid use for chronic pain management and an aging population. Digital adherence tools and combination therapies are emerging trends, enhancing patient compliance and quality of life. Germany’s emphasis on clinical evidence, regulatory support, and innovative drug launches positions it as a key European market in OIC treatment.
Analyst Opinion
A key driver of the opioid induced constipation treatment market is the surge in opioid consumption in North America and Europe. In 2024, U.S. opioid prescription rates reached approximately 46.7 prescriptions per 100 people, sustaining demand for effective OIC therapies. Pharmaceutical production capacity for OIC drugs expanded by an average of 12% annually between 2024 and 2026 to meet clinical needs.
Adoption of OIC therapies is growing beyond chronic pain management, extending into cancer-related palliative care. European oncology centers reported that nearly 60% of patients on opioid regimens experienced constipation by 2025, highlighting the need for specialized treatments that maintain analgesic efficacy.
Pricing strategies are evolving due to rising cost sensitivity among healthcare providers. From 2024 to 2026, OIC drug prices adjusted on average by 3–5% annually, balancing market accessibility with innovation costs and influencing overall market share.
Micro-level trends show increasing prevalence of OIC among aging populations, particularly in Asia Pacific, where geriatric patient numbers rose by 7% in 2025. This demographic shift drives the development of localized treatment formulations for elderly patients, illustrating how granular market dynamics impact broader industry trends.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 1.62 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 8.9% | 2033 Value Projection: | USD 2.95 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | AstraZeneca PLC, Pfizer Inc., Shionogi & Co, Ltd., Allergan, Cara Therapeutics, Inc., Nektar Therapeutics, Mylan N.V., Tarsa Therapeutics, Recro Pharma, Inc., Indivior PLC | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Opioid Induced Constipation Treatment Market Growth Factors
The global opioid-induced constipation treatment market is being driven by the rising prevalence of chronic pain, with diagnosed cases expected to grow by 30% from 2024 to 2026, increasing opioid prescriptions and the need for OIC therapies. Accelerated regulatory approvals in the U.S. and Europe, reducing drug approval timelines by nearly 15%, have enabled faster market entry. Innovations in targeted therapies, particularly PAMORAs, are improving treatment outcomes and driving adoption, especially in hospital pharmacy networks. Additionally, growing healthcare spending on geriatric symptom management, projected to rise over 8% annually in Asia Pacific, further supports market expansion.
Opioid Induced Constipation Treatment Market Development
In 2025, AstraZeneca introduced an extended-release peripherally acting mu-opioid receptor antagonist (PAMORA) for opioid-induced constipation, designed to provide prolonged relief and improve patient quality of life.
Key Players
Leading Companies of the Market
AstraZeneca PLC
Pfizer Inc.
Shionogi & Co. Ltd.
Allergan
Cara Therapeutics, Inc.
Mylan N.V.
Tarsa Therapeutics
Recra Pharma, Inc.
Indivior PLC
Competitive strategies in the opioid-induced constipation treatment market have focused on product differentiation and the approval of novel PAMORA therapies to improve efficacy and minimize side effects. In 2025, AstraZeneca launched a next-generation oral PAMORA, achieving a 20% increase in market share within its first year, supported by strong clinical evidence demonstrating enhanced patient outcomes. At the same time, Pfizer emphasized strategic collaborations with healthcare providers to expand the reach of its injectable formulations, improving treatment access and patient adherence, particularly across North America, and reinforcing its position in the growing OIC market.
Opioid Induced Constipation Treatment Market Future Outlook
The future of the opioid-induced constipation treatment market is poised for robust growth, driven by rising opioid use, aging populations, and expanding chronic pain and cancer care segments. Targeted therapies, particularly PAMORAs, are expected to dominate due to their clinical efficacy and minimal impact on analgesia. Innovation in combination therapies, digital adherence tools, and novel delivery methods will enhance patient compliance and treatment outcomes. North America will remain a key revenue contributor, while Asia Pacific is likely to witness the fastest growth due to increasing healthcare expenditure and geriatric populations. Strategic collaborations, regulatory support, and emerging market penetration will continue shaping industry dynamics.
Opioid Induced Constipation Treatment Market Historical Analysis
The opioid-induced constipation treatment market has experienced steady growth over the past decade, driven by increasing opioid prescriptions for chronic pain and palliative care. Historically, laxatives and stool softeners dominated therapy, providing symptomatic relief but limited efficacy. The introduction of peripherally acting mu-opioid receptor antagonists (PAMORAs) in the early 2010s marked a significant shift toward targeted treatments, offering improved outcomes without affecting analgesia. Market expansion was supported by hospital pharmacy networks, rising awareness of OIC among healthcare providers, and gradual adoption of digital adherence tools.
Sources
Primary Research Interviews:
Gastroenterologists
Pain Management Specialists
Clinical Pharmacologists
Healthcare Providers in Oncology
Databases:
IQVIA Pharmaceutical & Healthcare Data
Global Health Data Exchange (GHDx)
OECD Health Statistics
World Health Organization (WHO) Medicines & Health Products Data
Magazines:
Pharmaceutical Technology
Drug Development & Delivery
Pain Medicine News
HealthTech Magazine
Journals:
Journal of Pain and Symptom Management
Clinical Therapeutics
Digestive Diseases and Sciences
Journal of Opioid Management
Gastroenterology & Hepatology
Newspapers:
The New York Times (Health)
The Guardian (Health)
Financial Times (Healthcare)
The Hindu (Health)
Reuters Health
Associations:
World Health Organization (WHO)
International Pain Management Association
American Gastroenterological Association
U.S. Food and Drug Administration (FDA)
European Medicines Agency (EMA)
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients