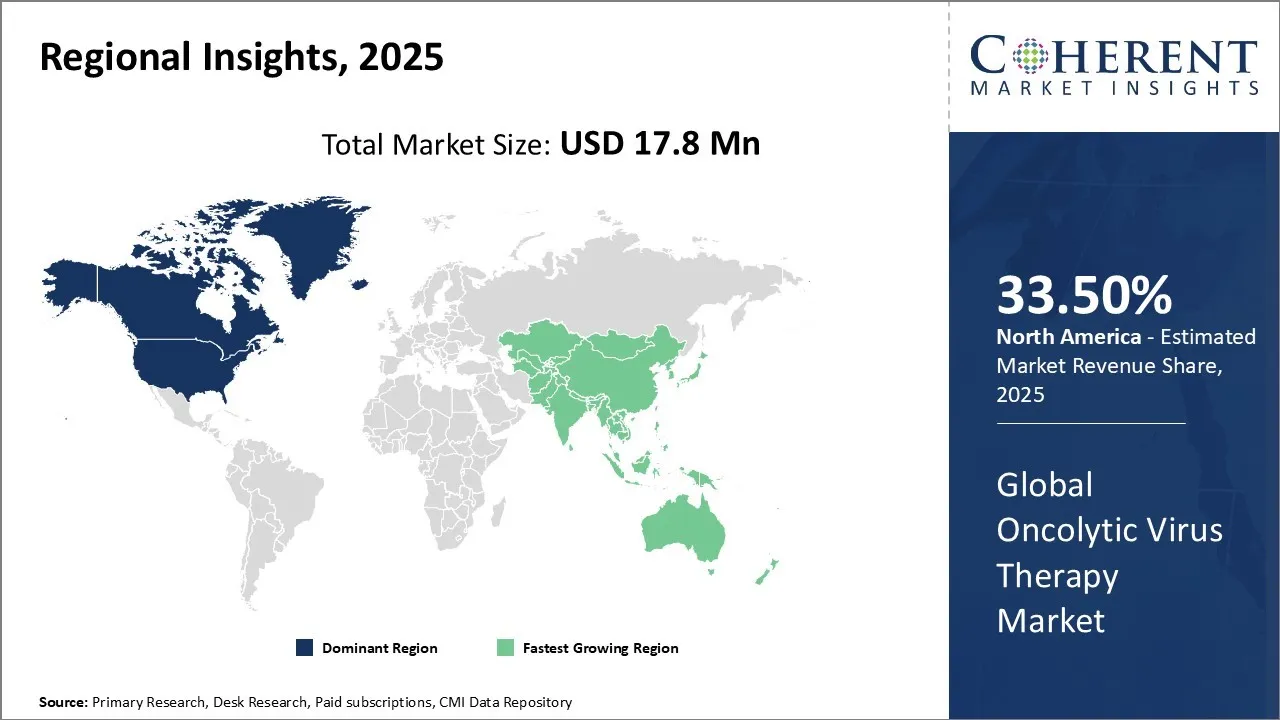
Oncolytic Virus Therapy Market is estimated to be valued at USD 17.8 Mn in 2025 and is expected to reach USD 87.3 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of25.5% from 2025 to 2032.
Oncolytic virus therapy market is gaining momentum as a next-generation cancer treatment, harnessing genetically engineered viruses to selectively infect, replicate within, and destroy cancer cells while sparing healthy tissues. This approach offers a dual mechanism, direct tumor cell lysis and immune system activation, making it particularly effective for hard-to-treat cancers such as melanoma, lung, breast, and pancreatic cancers. The growing oncolytic virus therapy market demand is being propelled by rapid advancements in viral vector engineering, deeper insights into tumor microenvironments, and increasingly positive clinical trial results.
|
Current Event |
Description and its Impact |
|
Regulatory Milestones and Designations |
|
|
Technological and Investment Trends |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
As of the latest available data, the OV therapy with broad market approval is Talimogene laherparepvec (T-VEC, marketed as Imlygic by Amgen), primarily indicated for the treatment of unresectable melanoma. The list price for Imlygic in the U.S. is approximately USD 65,000 to USD 80,000 per treatment course, depending on dosage and duration, though actual pricing may vary based on insurance coverage, negotiated discounts, and country-specific healthcare systems.
In terms of product type, the herpes simplex virus segment dominates the global oncolytic virus therapy market, holding an estimated share of 38.0% share in 2025, due to its unique biological and therapeutic characteristics that make it particularly suitable for cancer treatment. Herpes simplex virus has a large double-stranded DNA genome, allowing researchers to insert or delete genes to enhance tumor selectivity and immune response. Its genome can accommodate large transgenes, enabling the delivery of therapeutic payloads such as immune-stimulatory proteins (e.g., GM-CSF). HSV-based therapies not only kill cancer cells directly but also release tumor antigens that stimulate an anti-tumor immune response, effectively turning the tumor into a self-vaccine. Companies like Amgen, Replimune, and OncoVex continue to invest in HSV-based therapies for various cancers, including breast, head and neck, and pancreatic cancer. Numerous HSV-derived candidates are in Phase I to III clinical trials, expanding their relevance in the oncolytic virus therapy market.
In June 2024, Researchers at the Leibniz Institute of Virology (LIV), alongside international collaborators, visualized how herpesviruses exit the cell nucleus without damaging their envelope, which is a critical step for viral assembly and propagation. Using cutting-edge electron cryo‑tomography, the team mapped the flexible nuclear egress complex (NEC) of Herpes simplex virus 1 and a model pseudorabies virus in unprecedented detail. This was published in Nature Microbiology, and the findings offer a novel target for antiviral drug development. By disrupting NEC structural flexibility, future therapies may more effectively hinder herpesvirus replication, improving outcomes, especially for immunocompromised patients.
In terms of application, the melanoma segment is expected to command the largest share of the market in 2025. Oncolytic virus therapy is widely used in melanoma, as this type of cancer is highly responsive to immune-based treatments and presents several biological characteristics that make it an ideal target. Melanoma cells typically have a high number of genetic mutations, making them more recognizable to the immune system. Oncolytic viruses can infect and kill these cells, while also exposing tumor antigens that help the immune system mount a stronger, more targeted response.
Additionally, Numerous studies are exploring combinations of oncolytic virus therapy with other treatments like immune checkpoint inhibitors, especially in melanoma. Trials like MASTERKEY-265 (T-VEC + pembrolizumab) show promise in treating unresectable melanoma, reinforcing its central role in this field.
In December 2024, new data reveal that RP1 (vusolimogene oderparepvec), a genetically engineered HSV‑1 oncolytic virus, paired with nivolumab, achieved a 33% response rate in advanced melanoma patients previously treated with anti–PD‑1 therapy (NCT03767348). Notably, injections into deep visceral tumors such as liver and lung, proved safe and boosted responses to 42.9%, compared to 29.8% with skin-only injections. This is further proliferating the oncolytic virus therapy market share.
In terms of development stage, the pipeline phase, especially the phase I segment is expected to contribute the highest share of the market in 2025, the high volume of candidates, need for regulatory validation, and strategic investment focus make Phase I the most active and in-demand development stage in the current oncolytic virus therapy landscape. The field of oncolytic virotherapy is rapidly evolving, with a surge in new viral platforms such as herpes simplex virus (HSV), adenovirus, reovirus, and vaccinia. Many of these therapies are entering Phase I for the first time, as research labs and biotech firms validate their safety in human patients.
Since oncolytic viruses are live biological agents, regulatory agencies require extensive Phase I testing to assess their safety, dosing, and immune response. This makes early-stage trials essential and highly prioritized before advancing to efficacy-focused trials.
Additionally, Major partnerships between biotech startups, universities, and cancer research institutions are fueling an increase in early-phase trial launches. These collaborations often target niche cancers or rare tumors, which begin with Phase I trials to establish safety parameters.
In February 2024, a USD 500,000 grant from the Alliance for Cancer Gene Therapy (ACGT) has been awarded to Dr. E. Antonio Chiocca of Brigham and Women’s Hospital to advance a groundbreaking oncolytic virus therapy for glioblastoma. The award supports preclinical efforts to refine modified viruses that attack brain-tumor cells and activate anti-tumor immune responses. Glioblastoma, a lethal brain cancer affecting roughly 15,000 U.S. adults annually with average survival around eight months, has shown promising results in phase I trials led by Dr. Chiocca. With this support, he plans to optimize viral delivery and immunogenicity ahead of future clinical tests.
In terms of distribution channel, the hospital pharmacies segment is expected to contribute the greatest share of the market in 2025 due to specialized handling and storage. Oncolytic viruses are biologic agents that must be stored, prepared, and administered under strict sterile and temperature-controlled conditions. Hospital pharmacies are equipped with the infrastructure and trained personnel needed to manage such biologics safely.
Moreover, most oncolytic virus therapies such as Talimogene laherparepvec (T-VEC) for melanoma are administered intratumorally or intravenously, procedures typically performed in hospitals or specialized oncology centers, not retail or online settings.
Additionally, oncolytic therapies may cause immune responses, including fever or inflammation, they require close monitoring for adverse effects. Hospitals provide immediate medical support and lab services that are essential for patient safety during treatment.
In April 2025, Chinese oncology centers reported progress in clinical trials using a modified Newcastle disease virus for advanced tumors, administered intravenously in hospital settings with careful inpatient monitoring. The trial involved 23 patients, with most experiencing measurable tumor shrinkage and halted progression during the hospital-administered treatment phase.
To learn more about this report, Download Free Sample
North America, holding a share of 33.50% in 2025, is expected to dominate the global oncolytic virus therapy market. The region boasts advanced research infrastructure, academic-industry partnerships, and progressive regulatory support—factors fueling innovation and real-world implementation.
For instance, in March 2024, the U.S. Food and Drug Administration granted Fast Track designation to ImmVira’s HSV-based therapy MVR‑T3011, developed for recurrent or metastatic head and neck cancer following resistance to standard treatments. This milestone underscores the speed and seriousness with which regulatory bodies are treating oncolytic virus candidates in North America.
In addition, Genelux’s Olvi‑Vec, a vaccinia virus therapy targeting platinum-resistant ovarian cancer, also secured Fast Track status in late 2023, with Phase III trials underway to potentially broaden its use to other solid tumors. And the Moffitt Cancer Center initiated a clinical trial of MEM‑288 combined with the immunotherapy Opdivo for progressed non–small cell lung cancer patients, showcasing the growing integration of oncolytic platforms in hospital research settings.
These regulatory endorsements and high-profile trial launches reflect both the oncolytic virus therapy market and North America’s position as a global innovation hub, powered by clinical capabilities, funding, and forward-looking frameworks that continue to drive the field forward.
The Asia Pacific region, holding a share of 16.8% in 2025, is expected to exhibit the fastest growth in the global oncolytic virus therapy market. Asia Pacific is rapidly advancing, particularly in countries like Japan, China, South Korea, due to the growing focused research, supportive regulatory frameworks, and growing drug approvals. In China, over 60 clinical trials are underway exploring oncolytic viruses, which are heralded by scientists as a potential “next frontier” for affordable cancer care, costing as little as USD 140 per dose in some trials, with early evidence of tumor regression.
Meanwhile, Japan pioneered regulatory progress: in early 2025, the health ministry approved Teserpaturev (G47Δ), a genetically engineered herpesvirus for malignant glioma, achieving a one-year survival rate of 92.3% and showcasing hospital-based virotherapy integration. This landmark approval also reflects Japan’s conditional & fast-track regulatory pathways that accelerate access to novel biologics.
Additionally, countries like South Korea are fostering collaborations between biotech firms and academic institutions for next-generation cancer solutions, positioning the region as a hub for immuno-oncology innovation. This is further proliferating the oncolytic virus therapy market revenue.
The United States Acquires the prominent oncolytic virus therapy market share, backed by strong biotech investment, advanced clinical research, and regulatory support. It was the first to approve an oncolytic virus therapy which is Talimogene laherparepvec (T-VEC) for melanoma setting the stage for innovation. The U.S. continues to drive early-phase trials targeting solid tumors, melanoma, and pancreatic cancer, with active participation from biotech firms and academic centers he nation’s well-established regulatory pathways and active funding environment continue to encourage rapid development and commercialization of these advanced therapies, making the U.S. a central hub in the evolving global landscape of oncolytic virotherapy.
China is becoming a key player in oncolytic virus therapy due to its large patient base, cost-effective trials, and strong government support. In September 2024, Virogin Biotech’s VG161, an HSV-1–based therapy, received Breakthrough Therapy Designation from China’s CDE for liver cancer—the country’s first oncolytic virus to do so. In May 2025, VRT106, derived from hepatitis A virus, began first-in-human trials for solid tumors. With over 60 active clinical studies, China is advancing rapidly in the oncolytic virus therapy market through innovation and regulatory backing.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 17.8 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 25.5% | 2032 Value Projection: | USD 87.3 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Amgen Inc., Merck & Co., Inc., Oncolytics Biotech Inc., Circio Holding ASA, Akamis Bio, Vyriad, Inc., SillaJen Biotherapeutics, Cold Genesys Inc., Sorrento Therapeutics, Inc., Takara Bio Inc., Replimune Group Inc., Genelux Corporation, Synthetic Biologics, Inc., Lokon Pharma AB, and Elicera Therapeutics |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Oncolytic virus therapy market value stands at the frontier of precision oncology, not merely as an experimental adjunct, but increasingly as a transformative modality capable of reshaping standard cancer treatment protocols. In my professional assessment, the promise of oncolytic viruses lies not in their cytolytic capabilities alone, but in their profound immunomodulatory potential, an aspect that traditional chemotherapeutic and targeted agents have repeatedly failed to optimize in refractory malignancies.
Clinical data from recent trials underscores this shift. The Phase III trial of Talimogene laherparepvec (T-VEC) in unresectable melanoma demonstrated a durable response rate of 16.3%, a stark improvement compared to the 2.1% in the control arm. More importantly, T-VEC was observed to convert “cold” tumors into “hot” ones inducing an immune-permissive microenvironment marked by increased CD8+ T cell infiltration and PD-L1 expression. This characteristic alone opens substantial therapeutic value, especially when used in combination with checkpoint inhibitors.
Also, synergistic activity with immune checkpoint blockade is where the next-generation oncolytics are making definitive inroads. A Phase Ib study evaluating the combination of DNX-2401, an oncolytic adenovirus, with pembrolizumab in recurrent glioblastoma showed a 12-month overall survival rate of 52.7%, substantially higher than historical controls in this aggressive setting. These data suggest that oncolytic viruses are not simply direct tumoricidal agents—they are primers of systemic antitumor immunity.
Still, the platform faces two critical bottlenecks: delivery and selectivity. While intratumoral injection has shown promise, systemic delivery remains a challenge due to neutralizing antibodies and hepatic sequestration. However, innovations such as viral cloaking techniques, tumor-specific promoters, and engineered cytokine expression (e.g., GM-CSF, IL-12) are gradually mitigating these barriers. The recently launched LOAd703 platform in pancreatic and ovarian cancers is a case in point—leveraging a replication-competent adenovirus engineered with both immune-stimulatory and targeting genes.
*Definition: Oncolytic virus therapy is a cancer treatment approach that utilizes modified viruses to selectively target and destroy cancer cells while sparing healthy cells. These viruses are designed to replicate within tumor cells, leading to their destruction. Oncolytic virus therapy shows promise for various cancer types and aims to provide a more targeted and effective treatment option for patients.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients