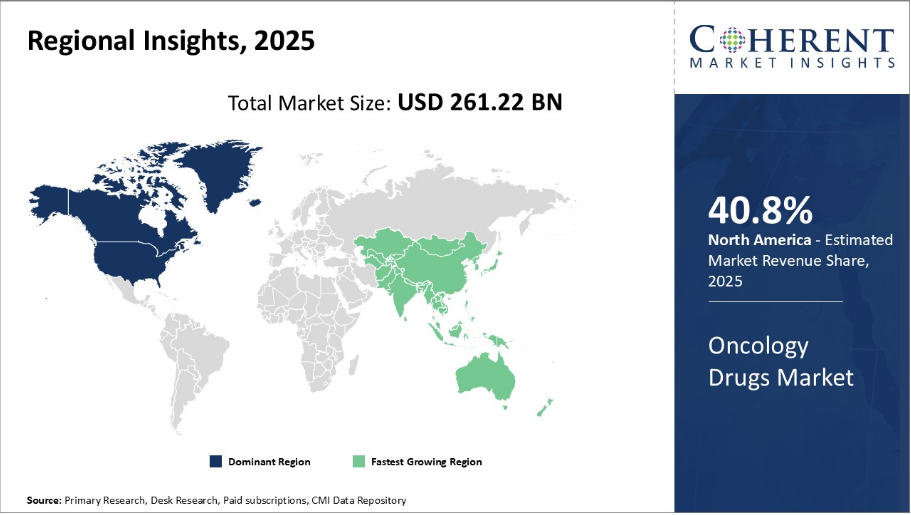
Oncology Drugs Market is estimated to be valued at USD 261.22 Bn in 2025 and is expected to reach USD 607.36 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 12.8% from 2025 to 2032.
To learn more about this report, Download Free Sample
The Oncology Drugs Market Size is growing quickly and is predicted to more than double in value over the next coming years due to innovation and the prevalence of cancer worldwide. The market is witnessing positive trends driven by the increasing prevalence of cancer worldwide. Advanced technologies in drug development have enabled better drug targeting and reduced side-effects. Besides this, rising healthcare spending and demand for affordable cancer treatments especially in developing nations provide attractive opportunities for market players.
|
Event |
Description and Impact |
|
Breakthroughs in Targeting “Undruggable” Cancer Proteins |
|
|
Widespread Adoption and Price Reduction of Generic Oncology Drugs |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The oncology drugs market is characterized by a robust and dynamic pipeline, with numerous therapies in various stages of development ranging from preclinical to late-stage clinical trials. The focus is on innovative approaches such as targeted therapies, immunotherapies, and antibody-drug conjugates (ADCs), which are transforming cancer treatment by offering higher efficacy and improved patient outcomes.
The pipeline is further strengthened by collaborations between pharmaceutical companies and academic institutions, as well as an increasing number of licensing agreements and mergers aimed at accelerating research and development. Regulatory agencies have also introduced accelerated approval pathways, enabling faster market entry for promising oncology drugs. The anticipated launch of these new therapies is expected to significantly expand the market in the coming years.
The patent landscape in the oncology drugs market is highly competitive, with major pharmaceutical companies actively seeking to protect their innovations through robust patent portfolios. Patent expirations of blockbuster oncology drugs have paved the way for biosimilars and generics, intensifying competition and driving down costs in some segments.
However, companies continue to invest heavily in Oncology Drugs Market Research to maintain exclusivity and extend the lifecycle of their products by developing next-generation therapies or new indications for existing drugs. The patent environment is also shaped by frequent litigation and strategic alliances, as firms seek to defend or challenge intellectual property rights in this lucrative market.
The distribution channel segment includes hospital pharmacies, retail pharmacies, and online pharmacies. Hospital pharmacies contributes the highest share of the oncology drugs market and is projected to hold 39.4% of the Oncology Drugs Market Share in 2025. The majority of oncology drugs are administered in hospitals either through intravenous infusions or as take-home oral medications following inpatient treatment.
Hospital pharmacies have well-established procurement systems that can stock the extensive formulary of high-cost cancer drugs needed across departments like medical oncology, radiation oncology, and surgical oncology. They also have specialized compounding facilities to prepare parenteral chemotherapy regimens under strict safety protocols.
As cancer centers upgrade facilities and hire more oncologists, inpatient and outpatient chemotherapy services within hospitals have rapidly expanded. The convenience of "one-stop" cancer care including pharmacy services encourages patients to source their ongoing treatment needs directly from hospitals.
Ongoing clinical trials of new drugs and combination regimens also occur primarily in hospitals. Their relationships with pharmaceutical suppliers further enable contractual agreements for discounted pricing and better inventory management. As a result, hospital pharmacies remain the central nexus for oncology drug distribution.
The drug type segment includes cytotoxic drugs, targeted drugs, hormonal drugs, and others. Targeted drugs segment is anticipated to hold 39.4% of the market share in 2025. Targeted therapies are designed to interfere with specific molecules involved in cancer cell progression and growth, resulting in fewer side effects than traditional chemotherapy.
Continuous progress in deciphering the molecular and genetic alterations that drive tumor development has enabled the design of an array of targeted agents. Areas witnessing major breakthroughs include cancer immunotherapies, PARP inhibitors for ovarian and breast cancers, BCR-ABL inhibitors for chronic myeloid leukemia, and therapies targeting EGFR and ALK mutations in lung cancer.
Major pharmaceutical companies have heavily invested in targeted drug R&D given their high selectivity and commercial potential. Notable drug approvals in recent years include Pfizer's Ibrance for breast cancer, Merck's Keytruda for various solid tumors, and Novartis' Kisqali.
The cancer type segment includes lung cancer, breast cancer, colorectal cancer, prostate cancer, blood cancer, bladder cancer, and others. Breast cancer contributes the highest share of the oncology drugs market and is projected to hold 24.3% of the market share in 2025. Breast cancer is the most commonly diagnosed cancer in women worldwide, with over 2 million new cases each year.
Extensive public awareness campaigns by prominent organizations such as Susan G. Komen and Think Pink have educated women on the importance of self-exams and mammography screening. As a result, breast cancer is often detected at early, more treatable stages compared to other cancer types. Developed nations have national mammography programs that screen a high proportion of women aged 50 to 69 years.
Examples include the Breast Cancer Screening Program in the U.K. and the National Breast and Cervical Cancer Early Detection Program in the U.S. Early detection coupled with the wide range of treatment options available, including surgery, radiation, chemotherapy, hormonal therapy, and newer targeted therapies, have significantly improved breast cancer survival rates over the past few decades.
To learn more about this report, Download Free Sample
North America has established itself as a dominant region in the global oncology drugs market and is projected to hold 40.8% of the market share in 2025. With a strong presence of leading biopharmaceutical companies and healthcare infrastructure, the U.S. alone accounts for over 40.5% of the market share. Companies based in the U.S. have been at the forefront of R&D for novel anti-cancer therapies. This has ensured early approvals and commercial launches of innovative oncology drugs.
Additionally, higher acceptance of premium-priced specialty drugs among patients and physicians has enabled companies to successfully penetrate the market with their new drug offerings. However, recently some pricing pressure has been witnessed due to initiatives to curb rising healthcare costs.
The Asia Pacific region has emerged as the fastest growing market for oncology drugs. Melanoma Targeted Oncology Drugs Market is also witnessing rapid expansion in this region, driven by rising incidence rates and the introduction of novel therapies. Improving accessibility to healthcare along with a rise in disposable incomes in nations, such as China and India, are supporting market expansion.
These countries also offer lower manufacturing and R&D costs, attracting biopharmaceutical players to boost the local production of generics and biosimilar through partnerships with local players. This is positively impacting the affordability of oncology treatment. Furthermore, increasing patient awareness about early detection and the management of cancer indicates long-term market potential.
The market for cancer medications in Europe is further distinguished by significant involvement in global clinical trials and partnerships between academic institutions and pharmaceutical corporations, which promote ongoing innovation and the creation of new treatments.
With continuous improvements in cancer research and a growing emphasis on early detection and individualized treatment plans, the market is still vibrant despite obstacles like complicated regulatory frameworks and pricing pressures, providing hope for better patient outcomes throughout the continent.
The U.S. market is also characterized by significant investment in artificial intelligence and advanced technologies to accelerate drug discovery and development, as well as a robust pipeline of both branded and generic oncology drugs. Major pharmaceutical companies, extensive clinical trial activity, and supportive healthcare infrastructure further reinforce the country's leadership in this sector.
The widespread adoption of generics has made cancer therapies more accessible and cost-effective for a large patient population. Indian pharmaceutical companies are also playing a key role in the global supply of affordable oncology drugs, both domestically and through exports to other low- and middle-income countries. This has positioned India as a vital player in the global oncology landscape, especially in the context of generic and biosimilar drug production.
The government’s focus on expanding healthcare coverage and supporting local pharmaceutical innovation has led to increased approvals and adoption of both domestic and international oncology drugs. China’s large patient population, coupled with a robust pipeline and increasing investment in research and development, is transforming the country into a key growth engine for the global oncology drugs market.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 261.22 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 12.8% | 2032 Value Projection: | USD 607.36 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
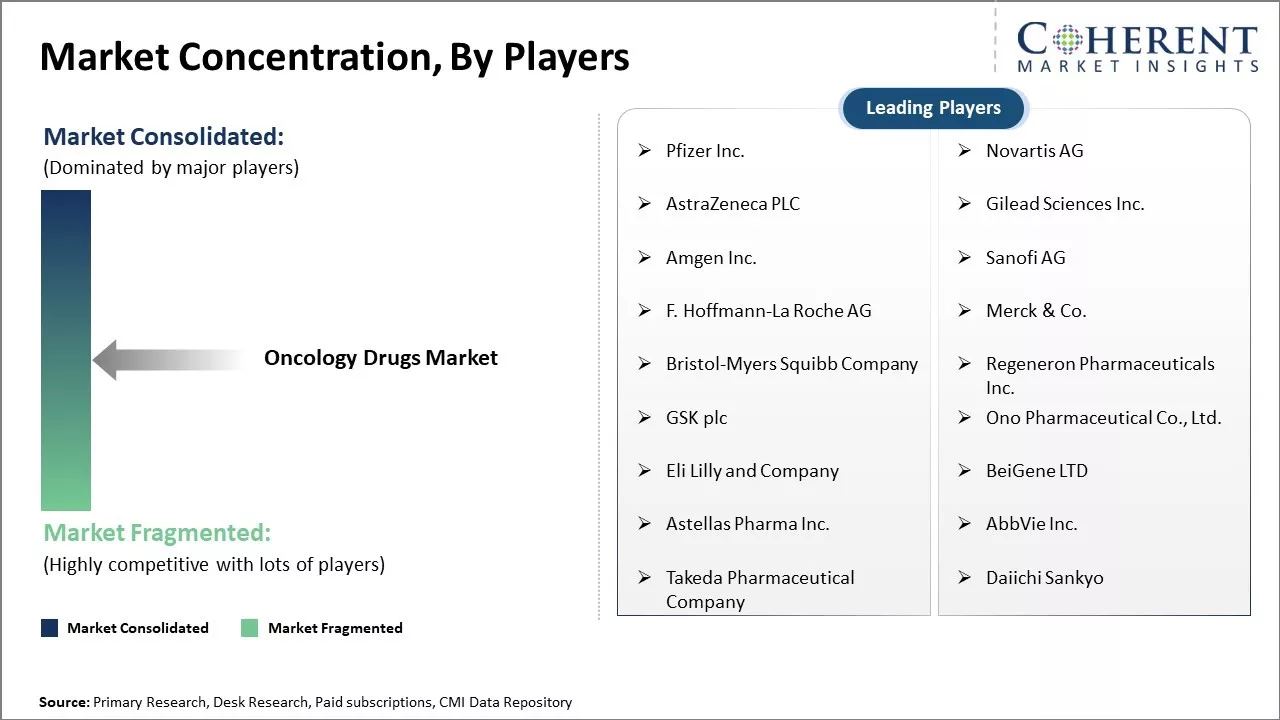
| Companies covered: |
Pfizer Inc., Novartis AG, AstraZeneca PLC, Gilead Sciences Inc., Amgen Inc., Sanofi AG, F. Hoffmann-La Roche AG, Merck & Co., Bristol-Myers Squibb Company, Regeneron Pharmaceuticals Inc., GSK plc, Ono Pharmaceutical Co., Ltd., Eli Lilly and Company, BeiGene LTD, Astellas Pharma Inc., AbbVie Inc., Takeda Pharmaceutical Company, and Daiichi Sankyo |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
To learn more about this report, Download Free Sample
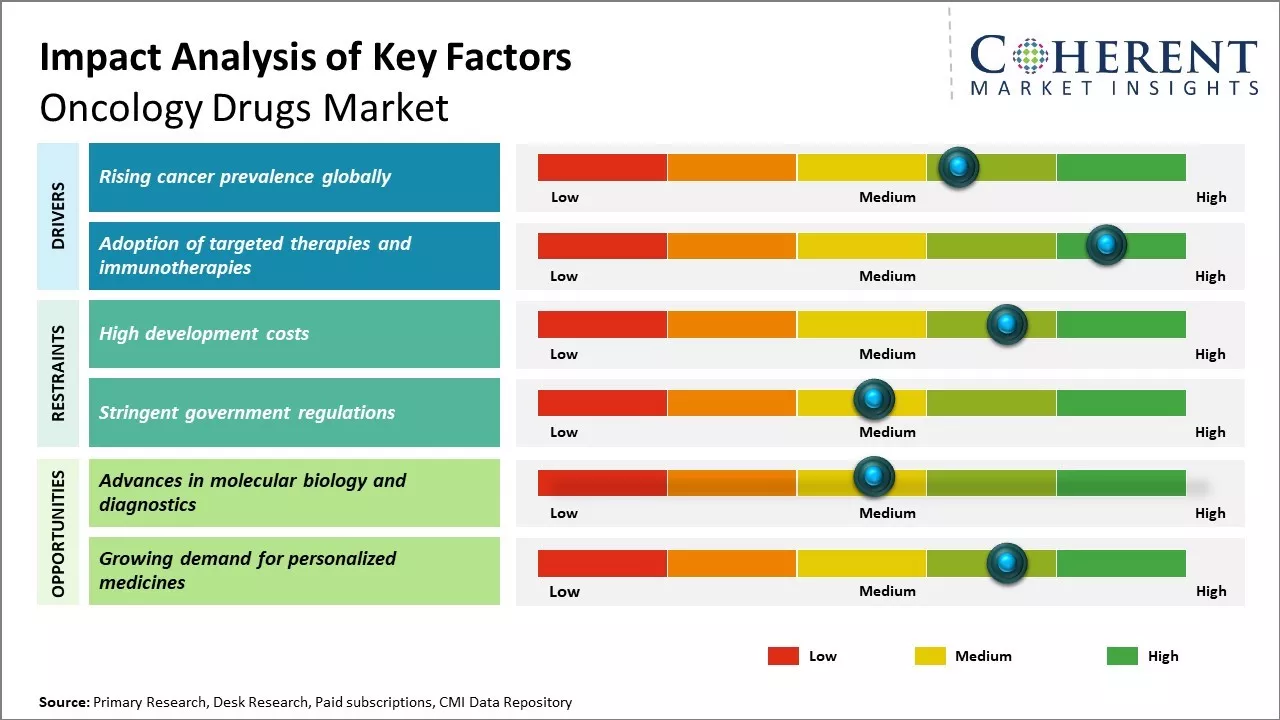
The prevalence of cancer cases has seen a consistent rise globally over the past few decades. Various factors such as growing elderly population, lifestyle changes, increasing exposure to pollution, and carcinogens have contributed to this rise. The International Agency for Research on Cancer (IARC), which is part of the World Health Organization (WHO), issued the estimates of the worldwide cancer burden.
According to the IARC, 20 million new cancer diagnoses and 9.7 million deaths occurred in 2022. The expected number of people who survived 5 years after a cancer diagnosis was 53.5 million. Cancer affects around one in every five persons in their lifetime, with one in every nine men and one in every twelve women dying from it.
With a rapidly ageing worldwide population susceptible to developing cancer, coupled with the prevalence of known causes such as smoking, alcohol use, etc., cancer diagnoses rates are expected to continue surging well into the future. This rising prevalence of cancer cases across the globe directly translates to the increasing demand for cancer treatments including oncology drugs.
While surgery and radiation continue to be important treatment modalities, pharmaceutical therapies have become the mainstay of treatment with the rapid advancement in targeted therapies and immunotherapies.
The oncology drugs market has witnessed a paradigm shift over the last decade with the introduction and rising adoption of targeted therapy and immunotherapy drug classes. While chemotherapy continues to play an important role, targeted therapies which impact specific molecular pathways driving cancer growth and immunotherapies which activate the body's immune system to fight cancer cells have transformed the treatment landscape.
Some key advantages of these newer therapies over chemotherapy include higher specificity leading to fewer side effects, prospect of long-term disease control and in some cases, potential for cure. Notable successes in clinical practice with drugs such as Herceptin, Gleevec, and Keytruda have boosted physicians’ confidence in these precision medicines.
There is also greater understanding among patients regarding the advantages of newer options compared to toxic chemotherapy regimens. As a result, the adoption rates of targeted therapies and immunotherapies are rapidly rising, especially in developed markets.
The oncology drugs market faces several challenges. High development costs and long approval times strain pharmaceutical company resources. As cancer treatments become more targeted and personalized, high costs may limit patient access. Generic competition threatens revenue for major drugs approaching patent cliffs. Despite increased understanding, cancer remains difficult to prevent and cure, necessitating continued research investment amid an increasingly difficult funding environment.
Improvements in molecular glues, targeted therapies, and customized medicine are also helping the industry since they enable more accurate and efficient treatments that cause the least amount of damage to healthy cells. While continuing patent expirations open the door to more competition and a wider patient base, the increasing availability of generic cancer medications is also boosting pricing and accessibility, especially in developing nations.
The market for oncology pharmaceuticals is predicted to continue to grow and innovate robustly over the next ten years despite obstacles like patent cliffs and regulatory barriers, as well as rising global cancer incidence.
An aging global population contributes to higher cancer incidence and creates Oncology Drugs Market Demand. Advances in molecular biology and diagnostics enable more precise targeting of cancer biology. Large patient populations and willingness to pay for breakthrough treatments support development in even rare cancers. Companion diagnostics and biomarkers could further personalize care. Growth areas include immunotherapy, combination therapies, and treatments for cancers with high unmet needs.
*Definition: Oncology drugs are medications specifically designed to treat cancer, which is a group of diseases characterized by the uncontrolled growth and spread of abnormal cells. These drugs encompass a broad range of therapies, including chemotherapy, targeted therapies, immunotherapies, and hormone therapies.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients