Ocular Drug Delivery Technology Market Size and Forecast – 2025 – 2032
The Global Ocular Drug Delivery Technology Market size is estimated to be valued at USD 6.8 billion in 2025 and is expected to reach USD 11.7 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 8.4% from 2025 to 2032. This steady growth underscores increasing adoption of innovative drug delivery platforms and expanding therapeutic areas propelled by rising prevalence of ocular disorders worldwide.
Global Ocular Drug Delivery Technology Market Overview
Ocular Drug Delivery Technology covers methods for delivering medicines directly to the eye, ranging from traditional drops and ointments to advanced sustained-release implants, injectable formulations, punctal plugs, and nanoparticle carriers. These systems are designed to improve drug absorption, extend release time, and reduce dosing frequency, providing more effective management of eye conditions such as glaucoma, macular degeneration, and diabetic retinopathy.
Key Takeaways
The sustained-release implant segment dominates the ocular drug delivery technology market share due to enhanced patient compliance and sustained therapeutic efficacy. The nano-formulation subsegment is forecasted to register the fastest growth, capitalizing on improvements in drug bioavailability.
The glaucoma application segment accounts for the largest market revenue, driven by the high prevalence and the need for chronic management solutions, while age-related macular degeneration displays significant growth potential due to demographic factors.
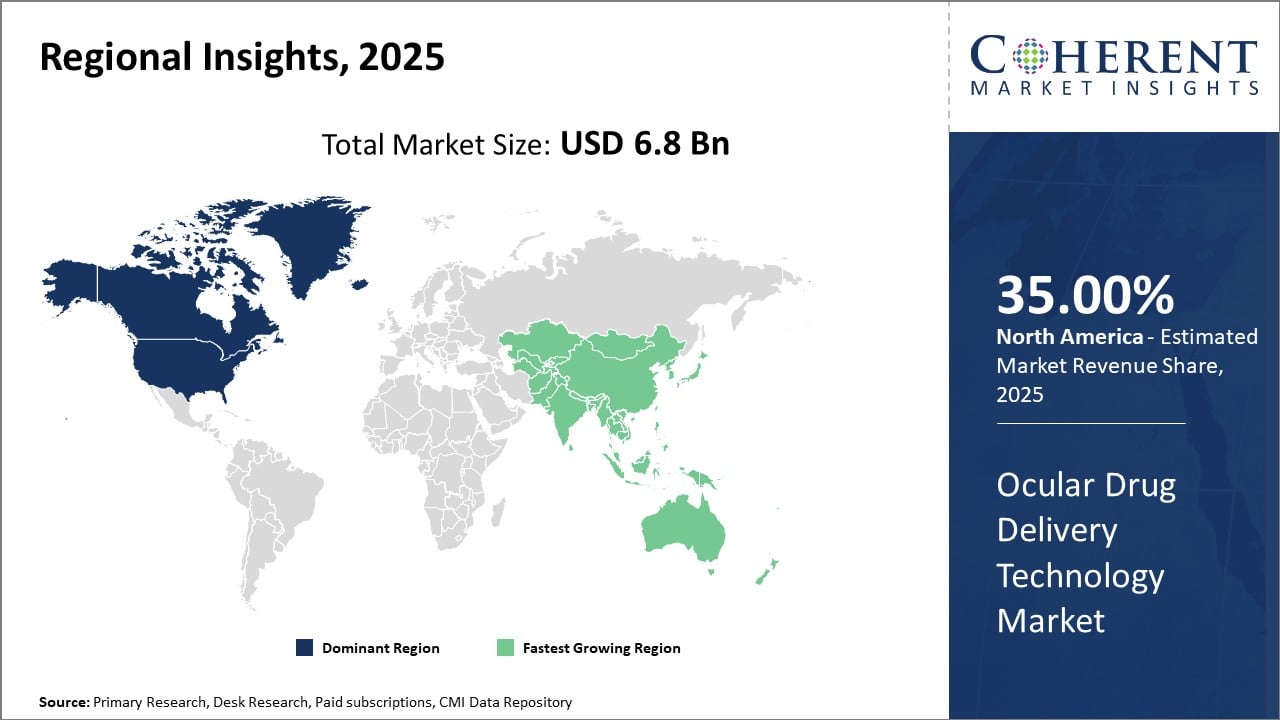
North America holds the leading regional share in the ocular drug delivery technology market, attributable to robust healthcare infrastructure, high adoption rate of advanced drug delivery systems, and significant R&D investments. Asia Pacific is the fastest-growing region owing to increasing healthcare expenditure, improving regulatory conditions, and growing disease burden.
Ocular Drug Delivery Technology Market Segmentation Analysis
To learn more about this report, Download Free Sample
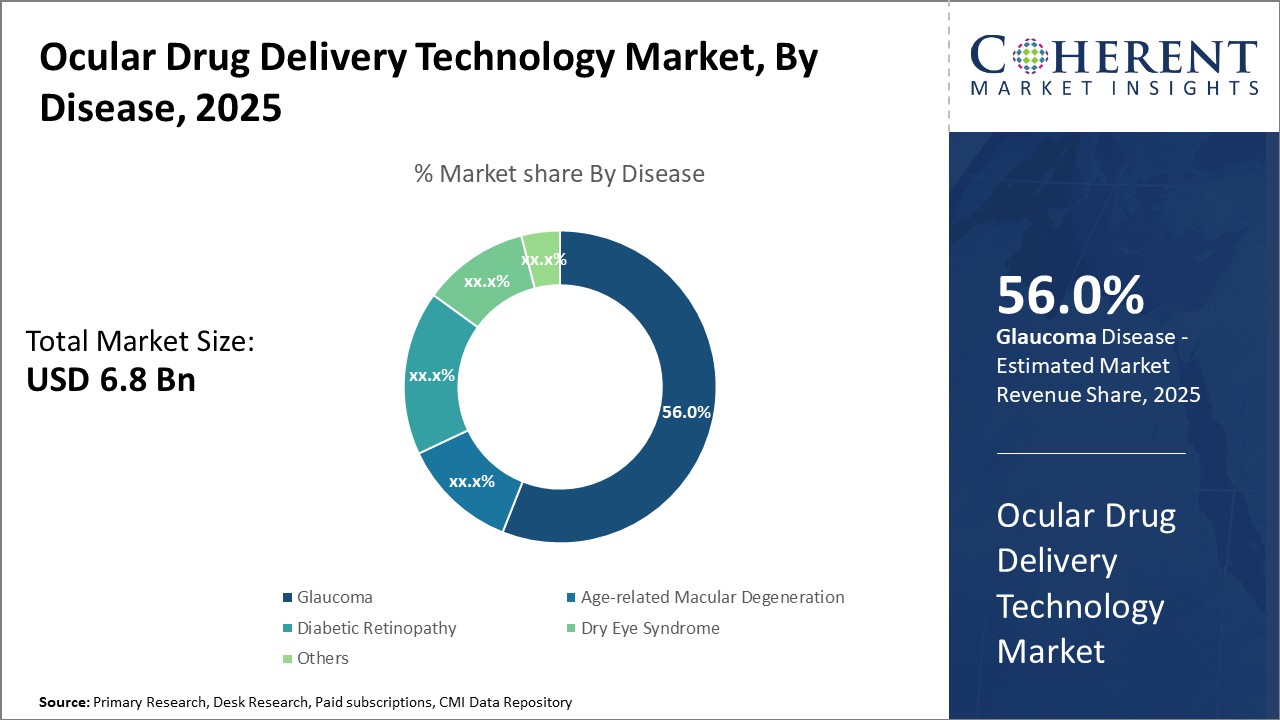
Ocular Drug Delivery Technology Market Insights, By Disease
In terms of application, the Glaucoma dominates the market share at 56%. This dominance is due to the high prevalence of glaucoma globally and the chronic nature of the diseas,e necessitating continuous drug delivery solutions for intraocular pressure control. The fastest-growing application subsegment is Age-related Macular Degeneration, with increasing elderly populations worldwide and advancements in targeted drug delivery, allowing focused treatment to slow disease progression.
Ocular Drug Delivery Technology Market Insights, By Technology
In terms of technology, the Sustained-Release Implants dominate the market share at 42%. This segment benefits from the ability to offer prolonged drug delivery over weeks or months, reducing dosing frequency and improving patient adherence, which is crucial for chronic ocular conditions such as glaucoma. The fastest-growing subsegment is Nano-formulations, driven by their capability to enhance drug solubility, permeability, and sustained release through nanocarriers like liposomes and nanoparticles, enabling more precise treatment outcomes.
Ocular Drug Delivery Technology Market Insights, By End-User
In terms of end user, Hospitals dominate the market share. Hospitals offer comprehensive treatment options, equipped with surgical facilities and specialists necessary for administering advanced ocular drug delivery systems including implants and injectables. Specialty Clinics are the fastest-growing segment due to their focus on ophthalmology and increasing outpatient procedures which leverage newer drug delivery technologies to optimize patient outcomes.
Ocular Drug Delivery Technology Market Trends
Recent market trends reveal intensified focus on developing biodegradable sustained-release ocular implants, driven by successful FDA approvals and increasing physician preference for minimally invasive solutions.
The growth in nanotechnology-based formulations is also noteworthy, substantiated by rising nanoparticle therapeutic launches that aim to improve drug targeting and reduce systemic side effects.
There is a visible pivot toward emerging markets where rising incidence of ocular disorders coupled with government initiatives on eye health creates an enabling ecosystem for advanced drug delivery technologies.
Ocular Drug Delivery Technology Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Ocular Drug Delivery Technology Market Analysis and Trends
In North America, the dominance in the Ocular Drug Delivery Technology Market is attributed to a mature healthcare infrastructure, high healthcare expenditure, and early adoption of advanced drug delivery platforms. The region commands over 35% market share, underpinned by concentrated industry presence, including leading companies such as AbbVie and Allergan. Regulatory support and reimbursement policies further reinforce market adoption, particularly in the U.S.
Asia Pacific Ocular Drug Delivery Technology Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth, propelled by increasing prevalence of chronic ocular diseases, improving healthcare access, expanding middle-class population, and government support for ophthalmic research. The region’s CAGR reflects growing adoption of innovative ocular drug delivery devices, incentivized by competitive manufacturing costs and targeted health campaigns. Notable contributions stem from companies like Santen Pharmaceutical expanding their footprint in India and China through localized development and distribution.
Ocular Drug Delivery Technology Market Outlook for Key Countries
USA Ocular Drug Delivery Technology Market Analysis and Trends
The USA’s ocular drug delivery technology market is characterized by significant clinical research activities and robust regulatory frameworks that expedite product approvals. Innovative platforms, including FDA-cleared sustained-release implants and nanoparticle formulations, contribute heavily to the country’s market revenue. Leading companies leverage partnerships with academic institutions and biotech firms to drive pipeline innovation.
India Ocular Drug Delivery Technology Market Analysis and Trends
India’s ocular drug delivery technology market is expanding rapidly owing to high disease prevalence, favorable demographic factors, and government initiatives such as the National Program for Control of Blindness. The market benefits from cost-effective clinical trials and growing capabilities in pharmaceutical manufacturing. Indian players alongside multinational companies like Santen Pharmaceutical and Ajanta Pharma are fostering technology transfer agreements, improving local production of implants and nanoformulations.
Analyst Opinion
Sustained-release implants are emerging as a key growth driver within the ocular drug delivery technology market due to their ability to provide consistent therapeutic levels over extended periods. For instance, in 2024, sustained-release corticosteroid implants accounted for over 28% of the market share in implant devices, reflecting expanding clinical acceptance as evidenced by FDA approvals and notable uptake in North America.
Nanotechnology-based ocular drug delivery platforms have shown considerable promise in enhancing drug permeation and retention time within the eye. Clinical studies from 2025 demonstrate that lipid-based nanoparticles improved drug bioavailability by over 35% compared to conventional eye drop formulations for glaucoma treatment, indicating strong demand among patients requiring chronic medication.
Increasing incidence of chronic ocular diseases, particularly age-related macular degeneration affecting more than 196 million globally in 2024, is contributing to the expanding drug delivery technology market size. These demographic trends are driving the demand for innovative ocular drug delivery devices capable of addressing complex pathologies with minimal invasiveness.
Price optimization and scalability remain critical supply-side indicators influencing market share dynamics, especially in emerging economies. Cost reductions in manufacturing of biodegradable polymer implants in 2024 resulted in a 12% decrease in average treatment cost, broadening accessibility and fostering market revenue growth in Asia Pacific.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 6.8 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.4% | 2032 Value Projection: | USD 11.7 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Alcon Inc, Bausch + Lomb, Allergan plc, Santen Pharmaceutical Co., Ltd., Novartis AG, F. Hoffmann-La Roche Ltd, Pfizer Inc., Regeneron Pharmaceuticals, Ophthalmic Technologies Inc., Lumenis Ltd., Ajanta Pharma Ltd., Sun Pharmaceutical Industries Ltd. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Ocular Drug Delivery Technology Market Growth Factors
The mounting prevalence of chronic eye conditions such as glaucoma and diabetic retinopathy is exerting considerable pressure on healthcare systems worldwide, propelling the need for more effective ocular drug delivery systems. Coupled with aging populations, particularly in regions like Europe and North America, these epidemiological trends boost market dynamics significantly. The technological evolution, including nanocarriers and polymeric implants, enhances clinical outcomes and patient compliance, addressing traditional drug delivery limitations. Regulatory approvals for novel ophthalmic drug delivery devices are also accelerating market penetration, as seen in the recent 2024 FDA clearances that facilitated faster commercialization. Additionally, increasing government-funded healthcare programs and rising health awareness in emerging economies are acting as enablers for business growth and expansion.
Ocular Drug Delivery Technology Market Development
In May 2025, the FDA approved Susvimo (a port-based, continuous delivery implant) for diabetic retinopathy, making it the first continuous-delivery option requiring only one refill every nine months in appropriate patients already responsive to anti-VEGF therapy.
In March 2025, Revakinagene taroretcel (brand name Encelto) was approved as the first cell-based encapsulated gene therapy for MacTel (macular telangiectasia type 2), administered via intravitreal implantation and expressing ciliary neurotrophic factor to support photoreceptors.
Key Players
Leading Companies of the Market
● Alcon Inc
● Bausch + Lomb
● Allergan plc
● Santen Pharmaceutical Co., Ltd.
● Novartis AG
● F. Hoffmann-La Roche Ltd
● Pfizer Inc.
● Regeneron Pharmaceuticals
● Ophthalmic Technologies Inc.
● Ajanta Pharma Ltd.
● Sun Pharmaceutical Industries Ltd.
Competitive strategies are increasingly relying on strategic partnerships and focused R&D investments. For example, Alcon’s collaboration with a leading biotech firm in 2024 accelerated development of peptide-based ocular implants, resulting in a 15% increase in their market share in North America. Similarly, Santen Pharmaceutical employed licensing agreements in 2025 to expand its biological drug portfolio targeting retinal diseases, boosting its footprint in Asia Pacific.
Ocular Drug Delivery Technology Market Future Outlook
The ocular drug delivery market is set for steady mid-single-digit to low-double-digit growth as demographic pressure (aging populations), rising prevalence of retinal diseases, and demand for longer-acting therapies drive adoption of implants, injectable sustained-release systems, and novel carriers (nanocarriers, in situ gels). Continued innovation will focus on reducing injection frequency, improving posterior-segment targeting, and combining drug+device solutions; regulatory complexity and the need for long-term safety data will remain meaningful hurdles, so partnerships between biotech, device firms and ophthalmology specialists will be critical to commercial success.
Ocular Drug Delivery Technology Market Historical Analysis
Ocular therapeutics historically relied on topical drops and intravitreal injections; however, limited bioavailability (tear washout, corneal barriers) and the need for frequent dosing made chronic eye diseases (AMD, diabetic retinopathy, glaucoma) challenging to manage. Over the past decade there’s been growing development of sustained-release implants, nanoparticle and formulation innovations, periocular injections, and device-assisted delivery methods to improve ocular bioavailability and patient adherence. This technological evolution has steadily expanded the addressable market for advanced delivery platforms
Sources
Databases:
PubMed (Ophthalmology Research Database)
National Eye Institute (NEI) Reports
Magazines:
Ophthalmology Times
Retina Specialist Magazine
EyeWorld
PharmaVoice
Journals:
Journal of Ocular Pharmacology and Therapeutics (Mary Ann Liebert)
The Ocular Surface (Elsevier)
Progress in Retinal and Eye Research
Newspapers:
The Wall Street Journal (Biotech & Pharma Section)
Financial Times (Healthcare)
The Economic Times (Pharma & Medical Devices)
The Times of India (Health)
Associations:
American Academy of Ophthalmology (AAO)
Association for Research in Vision and Ophthalmology (ARVO)
European Society of Retina Specialists (EURETINA)
International Council of Ophthalmology (ICO)
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients