North America Wound Debridement Products Market is estimated to be valued at USD 343.9 Mn in 2025 and is expected to reach USD 574.3 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 7.6% from 2025 to 2032.
Analysts’ Views on North America Wound Debridement Products Market:
Increasing number of geriatric population, increasing prevalence of diabetes are expected to drive the North America wound debridement products market over the forecast period. According to report shared by the U.S. Centers for Disease Control & Prevention on June 29, 2022, 37.3 million people in the U.S. have diabetes, which is 11.3% of the total the U.S. population. Out of these only 28.7 million cases are diagnosed while 8.5 million cases are not even diagnosed, which is equivalent to 23.0% of adults. Cases of diabetic foot ulcers is present in total 2.4% of hospitalized patients. Overall rate of mortality is 13% and the incidences of limb amputation is nearly 25%.
North America Wound Debridement Products Market – Driver
Prevalence of injuries and trauma in North America region
The cases of injuries and trauma are constantly rising in North America region. Those are more common with geriatric patients. Sometimes due to pre-existing disease conditions the healing of injuries are delayed, which may drive growth of North America wound debridement products market. For instance, according data shared by the National Safety Council, in 2021, there were 62 million preventable medically consulted injuries in the U.S. in 2021 while there were 224,935 preventable injury related deaths and these are increasing at rate of 11.9% per year.
Increasing launches of new products for wound debridement
Approval of new products by the U.S. Food and drug administration can increase demand, and hence drive growth of North America wound debridement products market. For instance on March 28, 2023 Vaporox, a U.S.-based medical device company received the U.S. Food and Drug Administration (FDA) approval for wound care medical device VHT-200. It is designed to deliver a unique combination of ultrasonic mist and concentrated oxygen to heal chronic wounds.
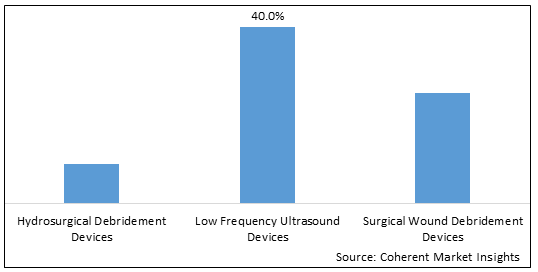
Figure 2. North America Wound Debridement Products Market Share (%), by Product Type, 2025
To learn more about this report, Download Free Sample
North America Wound Debridement Products Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others are facing problems with the transportation of drugs from one place to another.
However, the COVID-19 pandemic had a negative impact on the North America wound debridement products market due to reduction of patient visits to hospitals. For instance, according to data shared by the commonwealth fund on May 19, 2020, the number of patient visits to hospitals and ambulatory practices declined nearly 60% by the beginning of pandemic i.e. April 2020. Even after pandemic the rebound was occurred but number of visits were roughly one third lower than what was before the pandemic.
North America Wound Debridement Products Market Segmentation:
The North America wound debridement products market report is segmented into by product type, by application, and by end user.
Based on Product type, the North America Wound debridement products market is segmented into hydrosurgical debridement devices, low frequency ultrasound devices, surgical wound debridement devices, mechanical debridement pads, traditional wound debridement devices, larval therapy. Out of which, low frequency ultrasound devices are expected to dominate the North America wound debridement products market during the forecast period, and this is due to the increasing demand of ultrasonic devices of wound debridement management.
Based on Applications, the North America wound debridement products market is segmented into chronic ulcers, surgical wounds, traumatic wounds, burn cases. The chronic ulcers segment is expected to dominate the market over the forecast period and this is attributed to the increasing number of geriatric population in North America region.
Based on End user, the North America wound debridement products market is segmented into hospital, ambulatory surgical centers, specialized clinics, nursing facilities, others. The hospitals are expected to dominate the market over the forecast period and this is due to the increasing numbers of hospitals in North America region.
Among all segmentation, the product type segment has the highest potential due to the increasing approvals of wound debridement products by the key market players. For instance, on August 28, 2020, Tissue Regeneration technologies, LLC, a U.S.-based medical device company, received U.S. Food and drug administration approval for its product SoftWave, a tissue regeneration technology for treatment of burn wound.
North America Wound Debridement Products Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 343.9 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.6% | 2032 Value Projection: | USD 574.3 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Smith & Nephew plc, Zimmer Biomet, DeRoyal Industries, Inc., Lohmann & Rauscher International, Arobella Medical, LLC, Bactiguard AB, MediWound Ltd., Misonix, Inc., Söring GmbH, BSN Medical, and Derma Sciences Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
North America Wound Debridement Products Market: Key Developments
On November 5, 2020, Sanara Medtech, a U.S. based Biotechnology Company, launched Biakos, antimicrobial wound gel. It is hydrogel wound dressing that can be used with another product Biakos skin and wound cleanser to enhance effectiveness. Cleanser can clean wound and disrupt biofilm of microbes. It can remain on the wound for 72 hours. It can be used for diabetic foot ulcers, post-surgical wounds, pressure ulcers, first and second-degree burns, grafts and donor sites.
On April 8, 2021, Healogics LLC, a U.S.-based advanced wound care provider, launched healing awareness program on foot health called ‘The Healogics Foot Health campaign’ to prevent chronic wounds. In this program, people were made aware regarding need for regular foot screening to detect non-healing wounds. The team reached out to healthcare providers to encourage foot screening and provide patient focused educational resources. The program is dedicated to empowering both patients and healthcare providers with resources to help improve health, heal wounds and prevent amputations.
On March 8, 2023, Ceapro Inc., a Canada-based biotechnology company, announced that its study in collaboration with The Angiogenesis Foundation on Avena Sativa derived therapeutics β-Glucan and Avenanthramide was accepted for presentation at annual meeting of wound healing society. It was found that oats (Avena Sativa) can accelerate wound healing in mice by anti-inflammatory mechanisms and reduce scar formation. It can stimulate angiogenesis.
On January 1, 2022, Scapa Healthcare, a U.S.-based wound dressing company signed exclusive technology licensing agreement with Synedgen Inc., a U.S. based medical technology company for novel wound care technology. Under the terms of the agreement, Scapa Healthcare has exclusive rights over Synedgen’s glycopolymer technology in the field of dermal wounds and surgical care applications both over the counter and in the professional setting.
North America Wound Debridement Products Market: Key Trends
Awareness programs by key market players
On June 4, 2021, Sanara MedTech Inc., a U.S.-based surgical and chronic wound care product provider announced partnership with Pixalere Healthcare Inc., a Canada-based wound care Software Company, to advance its comprehensive wound and skin care strategy. The goal behind this partnership is to use Pixalere’s technology, including decision support, documentation, and wound tracking analytics, with complementary Sanara solutions that offer virtual access to expert wound and skin physicians, advanced diagnostics, and wound care product order fulfillment.
North America Wound Debridement Products Market: Restraint
Lack of trained manpower to handle wound debridement devices
Different devices such as hydro surgical debridement devices, low frequency ultrasound devices, surgical wound debridement devices, mechanical debridement pads, traditional wound debridement devices are being used in wound debridement procedures. However the proper training to operate these device is must. The lack of training programs inhibit the growth of North America wound debridement products market. For instance, according to article published by Journal of Clinical and Diagnostic Research for Doctors on March 1, 2021, the primary concerns are wound care management are nurse specialists lack training on new equipment and supplies as it is initially provided to surgeon doctors. There is also lack of trained professionals to guide patients in the usage of these devices for different conditions in the long-term treatment plan.
To counter balance the restrain, training programs should be conducted for all healthcare personnel as well as users.
High cost of wound care management
There is a high cost associated with wound care management. This is due to high cost associated with advanced wound dressing material, wound care devices and bioactive products. For instance according to article published by International Wound Journal on June 17, 2020, the gross healthcare cost per patient ranges from US$15,789 –US$ 17,761. This adds the cost of surgical dressings which are required to change every day. Apart from this the treatment formulation suggested by healthcare practitioner also adds up the cost. Depending on duration of treatment the cost increases.
To counter balance this problem, reimbursement policies should be introduced by government.
North America Wound Debridement Products Market - Key Players
Major players operating in the North America wound debridement products market include Smith & Nephew plc, Zimmer Biomet, DeRoyal Industries, Inc., Lohmann & Rauscher International, Arobella Medical, LLC, Bactiguard AB, MediWound Ltd., Misonix, Inc., Söring GmbH, BSN Medical, and Derma Sciences Inc.
Definition: Wound debridement is the process of removing dead tissue from wounds. It is necessary to accelerate healing process of the remaining healthy tissue. Biological debridement, enzymatic debridement, autolytic debridement, and mechanical debridement are some of the types of wound debridement. Hydrotherapy, wet-to-dry dressing, and monofilament debridement pads are the types of mechanical debridement.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients