Respiratory trainer devices are handheld devices that are designed to strengthen the muscles of expiration and inspiration. These devices are easy to handle and useful for anyone intending to improve the endurance of their breathing muscles. For individuals with inspiratory muscle weakness, the addition of inspiration muscle training to an exercise training program leads to improved performance.
The North America respiratory trainer market is estimated to be valued at US$ 190.2 Million in 2021 and expected to exhibit a CAGR of 7.3% during the forecast period (2021-2028).
Figure 1. North America Respiratory Trainer Market Share (%) in Terms of Value, by Product Type, 2021
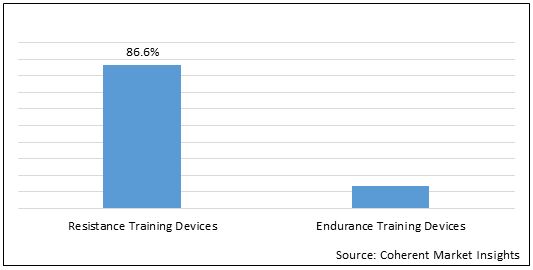
To learn more about this report, Download Free Sample
Increasing prevalence of respiratory disorders is expected to drive the North America respiratory trainer market growth over the forecast period.
The rising incidence of respiratory disorders is expected to drive the North America respiratory trainer market growth over the forecast period. For instance, according to the Asthma and Allergy Foundation of America in 2021, 25 million Americans have asthma each year. This equals to about 1 in 13 Americans, including 8% adults and 7% children, and about 20 million U.S. adults aged 18 years and older have asthma.
North America Respiratory Trainer Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2020 | Market Size in 2019: | US$ 190.2 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2021 to 2028 |
| Forecast Period 2021 to 2028 CAGR: | 7.3% | 2028 Value Projection: | US$ 311.1 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Teleflex Incorporated, Koninklijke Philips N.V., Smiths Medical, Inc., Vyaire Medical, Inc., IngMar Medical, POWERbreathe International Limited, PN Medical, Aleas Europe LLC, Aspire Products, LLC, and Airofit |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Figure 2. North America Respiratory Trainer Market Share (%), by Technique, 2021
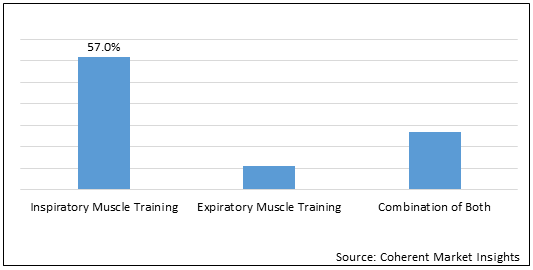
To learn more about this report, Download Free Sample
Increasing development and launches of novel respiratory trainer devices is expected to drive the market growth during the forecast period.
Key market players are focusing on the development and launch of novel respiratory trainer devices in order to expand their product portfolio and presence in the market. For instance, in August 2021, IngMar Medical, the world leader in breathing simulation, announced the launch of RespiPro, IngMar Medical's next-generation breathing and ventilation training solution. The solution includes a realistic breathing simulator, the ASL 5000, plus user-friendly software, realistic patient monitors, and a compact intensive care unit breathing mannequin.
North America Respiratory Trainer Market – Impact of Coronavirus (COVID-19) Pandemic
Supply chain and manufacturing activities in the U.S., Canada, and other North American countries have been disrupted due to lockdowns implemented by governments as well as delays in the transportation of raw materials. The coronavirus (COVID-19) pandemic and its consequent lockdown in various countries have impacted the financial status of businesses in all sectors.
The private healthcare sector is one of the sectors, which is majorly impacted by the COVID-19 pandemic. The lockdown has resulted in the closure of industrial establishments, except the manufacturing of essential commodities, and disruption in the supply chain of products. Thus, the COVID-19 pandemic has affected the economy in three main ways: 1) directly affecting the production and demand; 2) causing disruptions in distribution channels; and 3) causing financial impact on firms and the financial market.
However, as the prevalence of respiratory diseases increases with COVID-19 infection, the demand for respiratory trainers will also increase, which is expected to drive the North America respiratory trainer market growth over the forecast period. According to the World Health Organization (WHO), people with lung impairment after getting infected from COVID-19 should be rehabilitated by increasing their physical fitness and activity tolerance, which can be improved by respiratory muscle training (RMT).
North America Respiratory Trainer Market: Restraint
The major factors that hinder the North America respiratory trainer market growth include product recalls and critical regulatory compliance procedures. For instance, in July 2021, the U.S. Food and Drug Administration (FDA) directed Philips Respironics, a medical device company, to recall millions of sleep and respiratory devices following concerns that foam in the devices, which is used to reduce sound and vibration, may break into particles and enter the air hose of the device and be inhaled by the user. In response, the Military Health System (MHS) notified all TRICARE-authorized durable medical equipment providers about the recall, and MHS asked prescribing physicians to help notify patients of the recall and determine if they were issued a medical device on the Philips recall list.
Key Players
Major players operating in the North America respiratory trainer market include Teleflex Incorporated, Koninklijke Philips N.V., Smiths Medical, Inc., Vyaire Medical, Inc., IngMar Medical, POWERbreathe International Limited, PN Medical, Aleas Europe LLC, Aspire Products, LLC, and Airofit.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients