Peanut allergies are an overreaction of the immune system to peanuts. A peanut allergy is one of the most common types of food allergies. Infants with eczema and/or egg allergy are more likely to develop a peanut allergy. Among all food allergies, peanut allergy is the most common, and individual with a peanut allergy are at a greater risk for anaphylaxis
The North America, Europe, and Australia Peanut Allergy Treatment Market is estimated to be valued at US$ 1,252.6 million in 2022 and is expected to exhibit a CAGR of 11.7% during the forecast period (2022-2030).
Figure 1.North America, Europe, and Australia Peanut Allergy Treatment Market Share (%) in Terms of Value, By Drug Type, 2022
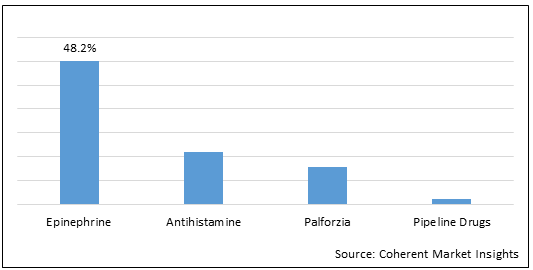
To learn more about this report, Download Free Sample
Increasing prevalence of peanut allergy is expected to drive market growth during the forecast period.
Increasing prevalence of peanut allergy is expected to drive growth of the North America, Europe, and Australia peanut allergy treatment market over the forecast period. For instance, According to a factsheet provided by Allergy UK, is a British medical charity dedicated to helping adults and children with their allergies, in July 2021, Peanut allergy affected around 2% of children in the UK. Peanuts are a common cause of food allergy. It usually develops in early childhood but, sometimes, peanut allergy can appear in adult age. Peanut allergy remains constant and only approximately 1 in 5 children outgrow their allergy by 10 years of age.
North America, Europe, and Australia Peanut Allergy Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: | US$ 1,252.6 Mn |
| Historical Data for: | 2017-2020 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 11.7% | 2030 Value Projection: | US$ 3,036.9 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
DBV Technologies, Aimmune Therapeutics, Inc., Prota Therapeutics Pty Ltd., COUR Pharmaceuticals, BlueWillow Biologics, HAL Allergy B.V., Allergy Therapeutics PLC., Novartis AG, Regeneron Pharmaceuticals, Inc., Vedanta Biosciences, Inc., Cambridge Allergy Ltd. (Camallergy), Angany Inc., Moonlight Therapeutics Inc., Allero Therapeutics B.V., Sanofi, Johnson & Johnson, Viatris Inc. (Mylan N.V.), Sun Pharmaceutical Industries Ltd., Bayer AG, Aurobindo Pharma Limited., Astellas Pharma Inc., Siolta Therapeutics, DESENTUM OY, ALK, Immunomic Therapeutics, Inc., Stallergenes Greer Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Figure 2.North America, Europe, and Australia Peanut Allergy Treatment Market Share (%), By Route of Administration, 2022.
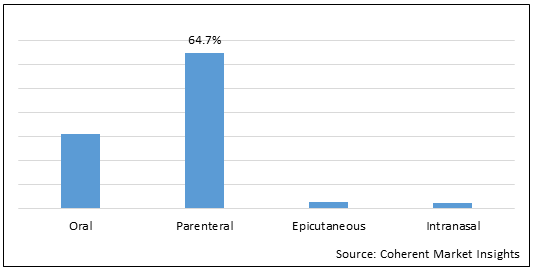
To learn more about this report, Download Free Sample
Increasing product approvals for treatment of peanut allergy are expected to drive market growth during the forecast period.
Increasing product approvals for treatment of peanut allergy are expected to drive the North America, Europe, and Australia Peanut Allergy Treatment Market growth during the forecast period. For instance, In January 2020, Aimmune Therapeutics, Inc., a biopharmaceutical company engaged in developing and commercializing treatments for potentially life-threatening food allergies, announced that the U.S. Food and Drug Administration (FDA) approved Palforzia [Peanut (Arachis hypogaea) Allergen Powder-dnfp]. Palforzia is the first approved treatment for patients with peanut allergy.
North America, Europe, and Australia Peanut Allergy Treatment Market– Impact of Coronavirus (COVID-19) Pandemic
Lockdown was imposed in several countries globally, which had a negative impact on the economy of the private healthcare sector globally. This lockdown resulted in the closure of industrial establishments, except manufacturing of essential commodities and disruption in supply chain of products. Thus, COVID-19 pandemic has affected the economy in three main ways: by directly affecting the production and demand, by creating disruptions in distribution channels, and by its financial impact on firms and financial markets. Supply chain and manufacturing activities in North America, Asia Pacific, Europe, and other global regions had been disrupted due to lockdown. Moreover, healthcare providers were facing challenges in terms of additional manpower, equipment, consumables, and other resources, which were required to ensure safety in hospitals and provide treatment to patients with other diseases, which has also impacted the overall healthcare market negatively. One of the biggest challenges is regarding the supply of raw materials required for manufacturing drugs due to irregularities in the transportation system. Furthermore, due to the increasing number of patients suffering from COVID-19 and other life-threatening illnesses, product distributors are seeing erratic demand for products from retailers.
North America, Europe, and Australia Peanut Allergy Treatment Market: Restraint
The high cost of peanut allergy drug is expected to hinder the growth of North America, Europe, and Australia peanut allergy treatment market over the forecast period. Palforzia is the only treatment approved by the U.S. Food and Drug Administration for children in age group of 4 to 17 years, allergic to peanuts. In January 2020, the U.S. FDA approved palfori allergan powder to mitigate allergic reactions, including anaphylaxis, which may occur with accidental exposure to peanuts. Palforzia costs from US$ 2.77 to US$ 35.60 per unit, depending on the different dosages (Powder for oral administration supplied in 0.5 mg, 1 mg, 10 mg, 20 mg and 100 mg Capsules or 300 mg Sachets.). Monthly cost of palforzia is around US$ 890 and an annual price of Palforzia is approximately US$ 11,000 per year. Thus, the high cost of peanut allergy drug makes it unaffordable for patients and this unaffordability of drug by patients is expected to hamper the market growth.
Key Players
Key players operating in market the market include DBV Technologies, Aimmune Therapeutics, Inc., Prota Therapeutics Pty Ltd., COUR Pharmaceuticals, BlueWillow Biologics, HAL Allergy B.V., Allergy Therapeutics PLC., Novartis AG, Regeneron Pharmaceuticals, Inc., Vedanta Biosciences, Inc., Cambridge Allergy Ltd. (Camallergy), Angany Inc., Moonlight Therapeutics Inc., Allero Therapeutics B.V., Sanofi, Johnson & Johnson, Viatris Inc. (Mylan N.V.), Sun Pharmaceutical Industries Ltd., Bayer AG, Aurobindo Pharma Limited., Astellas Pharma Inc., Siolta Therapeutics, DESENTUM OY, ALK, Immunomic Therapeutics, Inc., and Stallergenes Greer Ltd.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients