North America Companion Animal Drugs Market is estimated to be valued at USD 13.16 Bn in 2025 and is expected to reach USD 17.67 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 4.3% from 2025 to 2032.
Analysts’ views on North America Companion Animal Drugs Market:
An animal drug (also veterinary drug) refers to a drug intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease in animals. The U.S. Food and Drug Administration (FDA) has broad authority under the Federal Food, Drug, and Cosmetic Act to ensure the safety and effectiveness of veterinary drugs and its use in all animals, including farm animals. The FDA department responsible for this is the Center for Veterinary Medicine (CVM). Investigational new drug and new drug application analogs are known as investigational new animal drug and new animal drug, respectively. The FDA lists veterinary drug approvals in the FDA Green Book. FDA-approved drugs for veterinary use are structurally similar to drugs approved for humans in that the physiology is well conserved across species, and half of the drugs approved for animals are separately approved for human use.
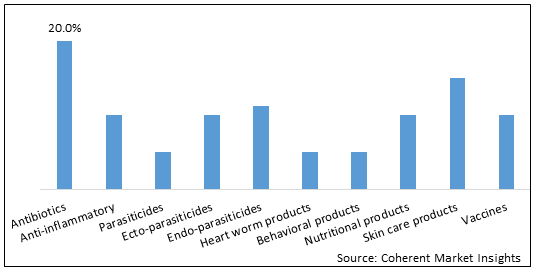
Figure 1. North America Companion Animal Drugs Market Share (%), By Product Type, 2025
To learn more about this report, Download Free Sample
North America Companion Animal Drugs Market– Drivers
Increasing government approvals in the animal drugs market
Increasing government approval in the animal drugs is expected to drive the North America companion animal drugs market growth over the forecast period. For instance, November 12, 2020 Zoetis Inc., an animal health company, announced that the European Commission has granted the company marketing authorization for Librela (bedinvetmab), the first injectable monoclonal antibody (mAb) therapy approved in the European Union for monthly alleviation of osteoarthritis (OA) pain in dogs. Librela provides veterinarians a new treatment option, which effectively controls OA pain for a month, while also offering a positive safety profile. Librela is the first-of-its-kind veterinary medicine that contains bedinvetmab, a monoclonal antibody that binds to Nerve Growth Factor (NGF), a key player in OA pain and in doing so reduces pain.
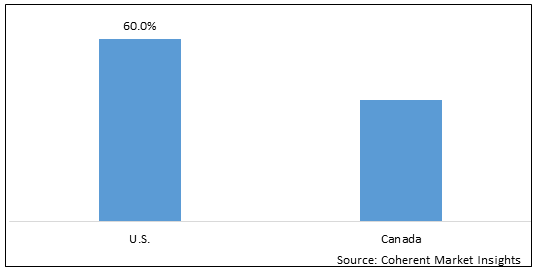
Figure 2. North America Companion Animal Drugs Market Value (US$ Million), By Country, 2025
To learn more about this report, Download Free Sample
North America Companion Animal Drugs Market - Country Analysis
Among country, U.S is estimated to hold a dominant position in the North America companion animal drugs market over the forecast period, owing to the increasing government approvals. For instance, on February 22, 2021 Zoetis Inc., an animal health company, announced that the European Commission has granted the marketing authorization for Solensia (frunevetmab), a new feline osteoarthritis treatment to alleviate pain. Osteoarthritis in cats is a very prevalent condition, existing in 40% of cats, and may generate pain and limit a cat’s comfort and quality of life, if not treated. Solensiaworks differently from current treatment options by targeting Nerve Growth Factor (NGF), a key player in osteoarthritis (OA) pain.
North America Companion Animal Drugs Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries, such as China, India, Saudi Arabia, the U.A.E., Egypt, and others faced problems regarding transportation of drugs from one place to another.
COVID-19 had negative impact on North America companion animal drug market due to drug side effects to animals. For instance, according to an article published by the U.S. Department of Health & Human Services, on August 26, 2021, stated during the COVID-19 pandemic, Ivermectin dispensing by retail pharmacies has expanded, as has the use of over-the-counter veterinary formulations that are not meant for human consumption. The U.S. FDA has issued a warning about the potential hazards of using some medications to treat or prevent COVID-19. Ivermectin is not authorized or approved by U.S. FDA for prevention or treatment of COVID-19. The National Institutes of Health’s (NIH) COVID-19 Treatment Guidelines Panel has also determined that there are currently insufficient data to recommend ivermectin for treatment of COVID-19. ClinicalTrials.gov external icon has listings of ongoing clinical trials that might provide more information about these hypothesized uses in the future. Adverse effects associated with ivermectin misuse and overdose are increasing, as shown by a rise in calls to poison control centers reporting overdoses and more people experiencing adverse effects.
North America Companion Animal Drugs Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 13.16 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 4.3% | 2032 Value Projection: | USD 17.67 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Zoetis Inc., Merck & Co., Inc., Bayer AG, Eli Lily & Co, Sanofi (Merial), Ceva Santé Animal, Virbac Animal Health, Boehringer Ingelheim GmbH |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
North America Companion Animal Drugs Market Segmentation:
North America companion animal drugs market report is segmented into product type, distribution channel, and region.
Based on product type, the North America companion animal drugs market is segmented into Antibiotics, Anti-Inflammatory, Parasiticides, Ecto-Parasiticides, Endo-Parasiticides, heart worm products, behavioral products, nutritional products, skin care products, and vaccines. Out of which, antibiotics segment is expected to dominate the market due to increasing research & development activities and easy for administration.
Based on distribution channel, the North America companion animal drugs market is segmented veterinary hospitals, veterinary clinics, pharmacies and drug stores. Out of which, veterinary hospitals segment is expected to dominate the market over the forecast period due to advanced treatments.
Based on country, the North America companion animal drugs market is segmented into U.S. and Canada. Among these, U.S. is expected to dominate the market over forecast period due to increased research & development activities.
Among the segmentation, product type segment is expected to dominate the market over forecast period. For instance, on January 16, 2020 Animal Health Institute stated pharmaceuticals include anti-inflammatory medications, anesthetics, pain medications, antibiotics, and specialized products for managing reproductive, cardiovascular, or metabolic conditions. To ensure effective and safe administration of animal pharmaceuticals, veterinarians and owners have a wide variety of options, including pills, liquids, injections, powders, feed additives, and boluses.
North America Companion Animal Drugs Market: Key Developments
On January 4, 2022 Zoetis Inc., animal health company announced that the U.S. Food and Drug Administration (FDA) has approved a new label indication for Simparica Trio (sarolaner, moxidectin, and pyrantel chewable tablets) for the prevention of Borrelia burgdorferi infections as a direct result of killing Ixodes scapularis vector ticks (black-legged or deer ticks). Simparica Trio is approved for dogs eight weeks of age and older weighing 2.8 pounds and greater. Simparica Trio is the first and only combination product demonstrated to prevent infections that may cause Lyme disease by killing deer or black-legged ticks.
On May 16, 2022 Ceva Santé Animale (Ceva), a multinational veterinary pharmaceutical company, acquired the Canadian oral rabies vaccine manufacturer Artemis Technologies, Inc. Artemis, located in, Canada, produces ONRAB, a rabies glycoprotein recombinant oral vaccine licensed for use in striped skunks (Mephitis mephitis) in Canada. The product also had positive field trial use in the USA in wild raccoons and skunks.
North America Companion Animal Drugs Market: Key Trends
Introduction of newer instruments for animal
Introduction of newer and more efficient instruments to boost up techniques can drive growth of market. For instance, On, June 1, 2021 Zoetis Inc., an animal health company, announced that it will expand its portfolio of equine horse care products, with the addition of Pro-Stride APS, Restigen PRP, and CenTrate BMA, a range of devices designed to help address injuries common in horses that may cause lameness.
North America Companion Animal Drugs Market: Restraints
Risk of treatment for animals
The risk of treatments for animals is expected to hamper the North America companion animal drugs market growth. For instance, on October 01, 2021, according to an article published by the American Veterinary Medical Association, U.S. Food and Drug Administration Center for Veterinary Medicine officials said in a letter to veterinarians and animal product retailers that people have become seriously ill from consuming highly concentrated ivermectin formulations, such as pour-on products, injectable products, pastes, and drenches meant for horses, cattle, and sheep. Agency officials also were receiving reports of local shortages of some veterinary-use approved ivermectin products, and they called for veterinarians and animal caretakers to report difficulties obtaining those drugs. It also called for veterinarians and retailers to report false claims about ivermectin and COVID-19. And they created a warning sign that veterinarians and retailers could post or distribute.
To counterbalance this restraint, a robust treatments should be introduced with lesser side effects.
North America Companion Animal Drugs Market- Key Players
Major players operating in the North America companion animal drugs market include Zoetis Inc., Merck & Co., Inc., Bayer AG, Eli Lily & Co, Sanofi (Merial), Ceva Santé Animal, Virbac Animal Health, and Boehringer Ingelheim GmbH.
*Definition: Veterinary medicine, also called veterinary science, is a medical specialty concerned with the prevention, control, diagnosis, and treatment of diseases affecting the health of domestic and wild animals and with the prevention of the transmission of animal diseases to people.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients