North America And Europe Dermal Filler Market is estimated to be valued at USD 2,540.4 Mn in 2025 and is expected to reach USD 4,052.7 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 6.9% from 2025 to 2032.
Analysts’ Views on North America and Europe Dermal Fillers Market :
The increase in geriatric population in the region of North America and Europe, increased healthcare expenditure and constant new approvals due to research and development activities are expected to drive the North America and Europe Dermal Fillers Market over the forecast period. For instance, in September 2022, U.S. Food and Drug Administration has approved Daxxify, manufactured by , a Nashville-Tennessee, U.S.-based aesthetics company. It is a new drug that may reduce the appearance of facial wrinkles for about six months, which can be an alternative to Botox.
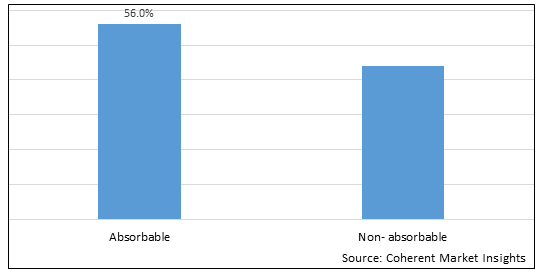
Figure 1. North America and Europe Dermal Fillers Market Share (%), by Product Type, 2025
To learn more about this report, Download Free Sample
North America and Europe Dermal Fillers Market – Driver
Increasing geriatric population
Increasing geriatric population is expected to propel growth of North America and Europe dermal fillers market over the forecast period. For instance, According to the U.S. Census Bureau in 2021, the American Community Survey estimated there were 55,892,014 people aged 65 and over in the U.S. out of a total population of 331,893,745 i.e. 16.8%. Also, according to data published on Eurostat in February 22, 2023, more than one fifth (21.1 %) of the European Union population was aged 65 and over in 2022. The median age of the EU’s population is increasing and was 44.4 years on January 1, 2022, meaning that half of the EU’s population was older than 44.4 years.
Increasing healthcare expenditure
Increasing healthcare expenditure is also expected to aid in growth of the market. For instance, according to Centers for Medicare & Medicaid Services, National Healthcare Expenditure (NHE) grew 2.7% to US$ 4.3 trillion in 2021, or US$ 12,914 per person, and accounted for 18.3% of Gross Domestic Product (GDP). Medicare spending grew 8.4% to US$ 900.8 billion in 2021, or 21 % of total NHE. According to the world bank, as per data retrieved on January 30, 2022 the Current health expenditure (% of GDP) of North America was 16.32 in 2019. While in Europe, healthcare expenditure is increasing, which results in growth of market. For instance, according to data published in Eurostat at February 27, 2023, General government expenditure in the EU on health amounted to US$ 129.39 billion or 8.1 % of GDP in 2021.
Launch of new products by key market players to expand product portfolio
With increasing demand of dermal fillers , key market players are focused on launching new products to expand their product portfolio. For instance, in December 22, 2022, the U.S. Food and Drug Administration (FDA) announced the approval of resilient Redensity produced by Teoxane laboratories, a Switzerland-based hyaluronic acid product manufacturing company.
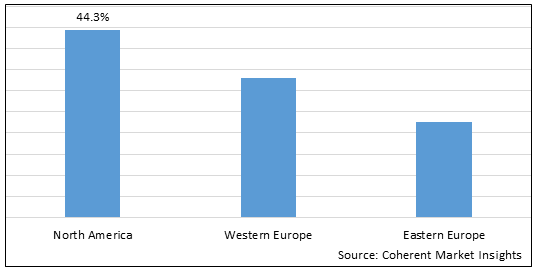
Figure 2. North America and Europe Dermal Fillers Market Value (US$ Million), by Region, 2025
To learn more about this report, Download Free Sample
North America and Europe Dermal Fillers Market - Regional Analysis
Among regions, North America is estimated to hold a dominant position in the Dermal Fillers Market over the forecast period. The growing numbers of surgeries directly impacts the increased demand for the dermal filler market. For instance, according to American Society of Plastic Surgeon report 2020, approximately 13.2 million cosmetic procedures were carried out in U.S., out of which, 3.4 million were soft tissue fillers. Thus, the rise in cosmetic surgeries is anticipated to increase market growth over the forecast period.
North America and Europe Dermal Fillers Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the manufacturing and distribution of products due to lockdown, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Also due to social distancing, the procedures were reduced. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others are facing problems with the transportation of drugs from one place to another.
The COVID-19 pandemic had a negative impact on the North America and Europe Dermal Fillers Market due to the reduced surgical procedures at hospitals during lockdown. Moreover, due to COVID-19 vaccination, there were several side effects such as hypersensitivity reactions were observed due to interaction of vaccine with dermal fillers. For instance, according to article published on March 16, 2022, delayed inflammatory reactions to dermal fillers was observed in response to mRNA COVID-19 vaccine by Moderna, a pharmaceutical and biotechnology company based in the U.K.
North America And Europe Dermal Filler Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 2,540.4 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.9% | 2032 Value Projection: | USD 4,052.7 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Galderma Pharma S.A., Sinclair Pharma plc., Allergan Plc., Anika Therapeutics Inc., Merz Pharma GmbH & Co. KGaA, Suneva Medical Inc., Teoxane Laboratories Inc., Prollenium Medical Technologies Inc., Adoderm GmbH, and Laboratoires Vivacy SAS. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
North America and Europe Dermal Fillers Market Segmentation:
The North America and Europe Dermal Fillers Market report is segmented into by Product Type, by Ingredients, by Distribution channels, and by Region.
By Product Type, the market is segmented into absorbable, and non-absorbable. Out of which, the absorbable segment is expected to hold a dominant position in the North America and Europe Dermal Fillers Market during the forecast period and this is attributed to the larger availability of these product as compared to non-absorbable one.
By Ingredient, the market is segmented into Hyaluronic acid, Poly-L- Lactic acid, Calcium Hydroxylapatite, Polymethylmethacrylate, Collagen. Out of which, hyaluronic acid segment is expected to hold a dominant position in North America and Europe Dermal Fillers Market during the forecast period and this is attributed to the increasing usage of hyaluronic acid in preparations of dermal fillers.
By Distribution channel, the market is segmented into retail pharmacies and drug stores, clinics and hospital pharmacies, and online sales. Out of which, the retail pharmacies and drug stores segment is expected to dominate the market over the forecast period and this is attributed to the preference by the users due to easy access and availability of products in retail pharmacy and drug store.
By Region, The market is segmented into North America, Western Europe and Eastern Europe. Out of which North America is expected to dominate the market over the forecast period and this is attributed to the country being economically stable and increasing preference by population for undergoing dermal filler procedures.
Among all the segmentations, the product type segment has the highest potential due to the increasing prevalence of absorbable products over the forecast period. Initiative by key market players to perform research and development activities of bioabsorbable products, more safe and efficacious process also contribute to growth of this segment. For instance, on February 8, 2022 Allergan Aesthetics, of , a U.S.-based biomedical company announced the U.S. Food and drug administration approval of Juvéderm Volbella XC for improvement of infraorbital hollows in adults over the age of 21.
North America and Europe Dermal Fillers Market Cross Sectional Analysis:
Launches of new online pharmacies in the Europe region are expected to boost demand for dermal fillers in these regions. For instance, in January 2021, the organic pharmacy, a London based wellness retail store which already have presence in Europe at Germany, Sweden, Denmark, and Portugal, opened a flagship concept store in Marylebon, U.K.
North America and Europe Dermal Fillers Market : Key Developments
Major players in the market are focused on adopting collaboration and partnership strategies to expand their product portfolio.
For instance, in January 2020, Revance Therapeutics, Inc. a U.S. based aesthetics company signed an agreement with TEOXANE SA, a U.K.-based COSMECEUTICAL company for distribution of TEOXANE SA’s Resilient Hyaluronic Acid technology in the U.S.
In January 2020, Histogen, Inc., a U.S.-based company, expanded its partnership with Allergan, Inc. U.S.-based pharmaceutical company, under which Allergan acquired exclusive rights to incorporate and commercialize Histogen's cell conditioned media in microdermabrasion therapies and exclusive rights to new Histogen intellectual property in the aesthetic field.
North America and Europe Dermal Fillers Market : Key Trends
Approval of newer products and technological advancements can drive the growth of market
Due to improved healthcare infrastructure, effective government policies, a sizable base of multinational corporations, the Dermal Fillers Market is expected to experience considerable expansion in the North American and Europe region.
For instance, in February 15, 2023, Neauvia organic fillers manufactured by Matex Lab Spa, a Italy-based company, scored CE marking under the European Union’s new Medical Device Regulation (MDR) for its range of facial dermal fillers products.
In October 2021, U.S. Food and Drug Administration approved , product of Prollenium, a Canada-based medical technology company. It is a new HA dermal filler product was officially launched onto the U.K. market. It reduces irritation, pain and swelling due to their smaller surface area, when used in minimum volume. The filler also undergoes a purification process to remove impurities after being wet milled and sieved to produce a uniformly cross-linked gel that is designed to degrade at an even rate.
North America and Europe Dermal Fillers : Restraint
High Cost of dermal filler syringes
High cost of dermal filler syringes and physician services for procedures as well as the need for multiple sessions for desired results is expected to hinder growth of North America and Europe dermal fillers market. For instance, according to American Society of Plastic Surgeon 2023, the cost of a syringe varies from US$ 717 to US$ 2,508. Also, patients need multiple syringes to achieve desired results. The service cost of physicians or surgeons is around 40 to 50% of the dermal filler cost. The fillers such Hyaluronic acid, Poly-L-Lactic acid, Calcium hydroxylapatite, collagen are biodegradable and last for 3 to 24 months. Most of them are naturally present in body and therefore, get degraded quickly. This in turn requires multiple sessions for attaining the desired results, which further increases the overall cost of the procedure.
Availability of alternative methods
Majority of the dermal fillers currently available are absorbable / bio degradable except for Poly(methyl methacrylate) (PMMA) fillers. Biodegradable fillers last for 3 to 24 months, whereas, PMMA fillers last for 4-5 years. This makes the procedure repetitive after the certain time, which may lead to a shift towards surgical facial rejuvenation instead of dermal fillers, which limits growth of the market. According to a blog published in Aedit, an online Aesthetic resource on May 8, 2020, Facial Fat transfer can also be an alternative to dermal fillers.
North America and Europe Dermal Fillers Market - Key Players
Major players operating in the North America and Europe Dermal Fillers Market include Galderma Pharma S.A., Sinclair Pharma plc., Allergan Plc., Anika Therapeutics Inc., Merz Pharma GmbH & Co. KGaA, Suneva Medical Inc., Teoxane Laboratories Inc., Prollenium Medical Technologies Inc., Adoderm GmbH, and Laboratoires Vivacy SAS.
*Definition: Dermal fillers, also known as injectable fillers or soft tissue fillers, are categorized as medical devices by the U.S. FDA. Dermal fillers are used to reduce wrinkles and smoothen skin appearance caused by ageing, acne or scars. The application areas are face including Nasolabial fold, cheeks, lips, corners of the eye, and dorsal area of hands.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients