North America and Europe Age-related Macular Degeneration Therapeutics Market is estimated to be valued at USD 14.07 Bn in 2025 and is expected to reach USD 21.3 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 6.1% from 2025 to 2032.
Analysts’ views on North America and Europe age-related macular degeneration therapeutics market:
Increasing prevalence of age related macular degeneration, increasing geriatric population, new product launches, and strategies like mergers, acquisitions, and collaboration are expected drive the North America & Europe age-related macular degeneration therapeutics market growth over the forecast period. For instance, according to the data published by the Centers for Disease Control and Prevention, on October 31, 2022, in 2019, an estimated 19.8 million (12.6%) Americans aged 40 and older were living with age-related macular degeneration (AMD). Of these, 1.49 million (0.94%) were living with vision threatening AMD. Prevalence of AMD increased with age from 2% among people aged 40 to 44 to 46.6% among people aged ≥85. By state, the crude prevalence of AMD ranged from a low of 6.2% in the District of Columbia to a high of 18.3% in Florida. Such high prevalence increases need of age-related macular degeneration therapeutics.
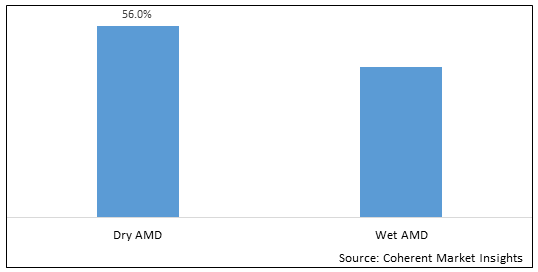
Figure 1. North America & Europe Age-Related Macular Degeneration Therapeutics Market Share (%), By Disease Type, 2025
To learn more about this report, Download Free Sample
North America and Europe Age-Related Macular Degeneration Therapeutics Market– Drivers
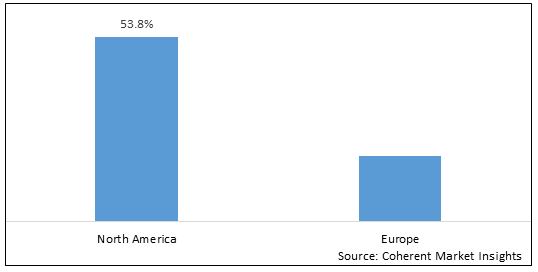
Figure 2. North America & Europe Age-Related Macular Degeneration Therapeutics Market Share (%), By Region, 2025
To learn more about this report, Download Free Sample
North America & Europe Age-Related Macular Degeneration Therapeutics Market- Regional Analysis
Among region, North America is estimated to hold a dominant position in the North America & Europe age-related macular degeneration therapeutics market over the forecast period, owing to increasing launches of products. For instance, on April 7, 2021, LumiThera, Inc., a U.S.-based commercial stage medical device company delivering photobiomodulation (PBM) treatment for ocular disorders and disease, announced positive findings in its LIGHTSITE II, multi-center clinical trial in dry Age-Related Macular Degeneration (AMD) patients. The prospective, double-masked, randomized, multi-center, clinical trial, titled LIGHTSITE II, was conducted in eight leading retinal centers based in the U.K., Germany, Spain, Italy, and France. The objective was to treat dry AMD subjects over the course of three rounds of PBM sessions every four months with a duration of 10 months. Results showed a steady improvement over time in BCVA measurements from baseline with the shortened PBM treatment intervals. Previously, LIGHTSITE I study results demonstrated some remission of visual benefits between PBM treatments delivered every six months. The benefits of four-month treatment intervals were more consistent in maintaining vision outcomes.
North America and Europe Age-Related Macular Degeneration Therapeutics Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries, such as China, India, Saudi Arabia, the U.A.E., Egypt, and others faced problems regarding transportation of drugs from one place to another.
However, the COVID-19 pandemic had negative impact on the North America & Europe age-related macular degeneration therapeutics market, due to reduced patient’s visits to hospitals. According to an article published by the Journal Current Opinion in Pharmacology on April 14, 2022, the COVID 19 affected negatively on retinal degenerative diseases. The complications in eye treatment were increased and this was due to reduced visits of patients. Delayed treatment due to fear, lifestyle, or as an adverse effect of COVID-19 is linked with visual acuity.
North America & Europe Age-Related Macular Degeneration Therapeutics Market Segmentation:
North America & Europe age-related macular degeneration therapeutics market report is segmented into disease type, drug type, distribution channel, and region.
Among all segmentation, the disease type segment has the highest potential due to increasing prevalence of disease with increasing geriatric population. For instance, according to data published by Fighting Blindness Canada on August 24, 2022, Age-related macular degeneration (AMD) is the leading cause of vision loss in people over the age of 55, affecting approximately 2.5 million Canadians. Each year in Canada, there are nearly 200,000 newly diagnosed cases of AMD.
North America and Europe Age-Related Macular Degeneration Therapeutics Market Cross Sectional Analysis:
Among disease type segment, dry AMD segment is dominant during the forecast period in Europe region due to increasing launch of newer treatments. For instance, on February 24, 2023, STADA Arzneimittel AG, a Germany-based pharmaceutical company, and Xbrane Biopharma, a Sweden-based biopharmaceutical company specialized in high demand biosimilars and long acting injectables, have announced that the U.K’s Medicines and Healthcare products Regulatory Agency (MHRA) has granted a marketing authorization for Ximluci, a biosimilar referencing ophthalmic drug, Lucentis (ranibizumab). Ranibizumab is a monoclonal antibody fragment created from the same parent mouse antibody as bevacizumab. It inhibits angiogenesis (the formation of new blood vessels) by inhibiting vascular endothelial growth factor A (VEGF-A). Ranibizumab can be used to treat macular degeneration by inhibiting VEGF.
North America & Europe Age-Related Macular Degeneration Therapeutics Market Report Coverage:
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 14.07 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.1% | 2032 Value Projection: | USD 21.3 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Novartis AG, Bayer AG, Bausch Health Companies Inc., Regeneron Pharmaceuticals, Inc., F. Hoffmann-La Roche AG, Pfizer, Inc., Valeant Pharmaceuticals International, Inc., AbbVie Inc., Viatris Inc. and Amgen Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
North America and Europe Age-Related Macular Degeneration Therapeutics Market: Key Developments
On October 22, 2021, F. Hoffmann-La Roche Ltd, a Switzerland-based multinational healthcare company, announced that the U.S. Food and Drug Administration (FDA) has approved Susvimo (ranibizumab injection) 100 mg/mL for intravitreal use via ocular implant for the treatment of people with neovascular or “wet” age-related macular degeneration (nAMD) who have previously responded to at least two anti-vascular endothelial growth factor (VEGF) injections. Neovascular AMD is a potentially blinding condition that requires treatment with eye injections as often as once a month. Susvimo, previously called Port Delivery System with ranibizumab, is the first and only U.S. FDA-approved treatment for nAMD that offers as few as two treatments per year.
On March 31, 2022, Novartis AG, a Switzerland-based global healthcare company, announced that the European Commission (EC) has approved Beovu (brolucizumab) 6 mg for the treatment of visual impairment due to diabetic macular edema (DME). Approval in DME represents the second indication for Beovu granted by the EC, which was first approved for the treatment of wet age-related macular degeneration. The EC decision applies to all 27 European Union (EU) member states as well as Iceland, Norway, and Liechtenstein. The EC approval was based on year one data from the Phase III, randomized, double-masked KESTREL and KITE* studies, which met its primary endpoint of non-inferiority in change in best-corrected visual acuity (BCVA) from baseline versus aflibercept at year one. In both trials, following the loading phase, over half of patients (55.1% in KESTREL and 50.3% in KITE) in the Beovu 6 mg arm remained on a 12-week dosing interval through year one. Aflibercept dosing was aligned to the approved EU label in year one of treatment
On May 13, 2020, Boehringer Ingelheim International GmbH, a Germany-based pharmaceutical company, and CDR-Life Inc., a Switzerland-based biotechnology company, announced they have entered into a collaboration and licensing agreement to research and develop antibody fragment-based therapeutics for geographic atrophy (GA). GA is a progressive, irreversible retinal disease that occurs in patients with age-related macular degeneration (AMD) for which there is no current treatment. Together, with Boehringer Ingelheim’s expertise in the therapeutic development of biologics and CDR-Life’s strong know-how in antibody engineering, the two companies will progress CDR-Life’s preclinical candidate, with the aim to preserve sight for patients with GA.
On January 27, 2022, Biogen, a U.S.-based global biotechnology company, announced that they have entered into an agreement whereby Samsung Biologics, South Korea based biotechnology company will acquire Biogen’s equity stake in the Samsung Bioepis joint venture for an aggregate consideration of up to US$ $2.3 billion. Upon the acquisition of Biogen’s stake, the companies will continue with its exclusive agreements, including commercialization of its current portfolio. This includes marketed products BENEPALI (etanercept), a biosimilar referencing ENBREL, IMRALDITM (adalimumab), a biosimilar referencing Humira, and FLIXABI (infliximab), a biosimilar referencing Remicade. Additionally, Biogen will also retain commercial rights for BYOOVIZTM (ranibizumab-nuna), an approved biosimilar referencing LUCENTIS (ranibizumab), as well as an investigational biosimilar candidate in development, SB15 (aflibercept), a proposed biosimilar referencing EYLEA.
North America and Europe Age-Related Macular Degeneration Therapeutics Market: Key Trends
Research collaboration between key market players
Research collaboration between key market players to boost up development of newer therapeutics can drive growth of market. On June 29, 2021, Ocular Therapeutix, Inc., a U.S.-based biopharmaceutical company focused on the formulation, development, and commercialization of innovative therapies for diseases and conditions of the eye, and Mosaic Biosciences, a U.S.-based biotechnology company, entered into a discovery collaboration to identify new targets and therapeutic agents aimed at the treatment of Dry Age-related Macular Degeneration (dAMD). Under terms of the agreement, the collaboration between Ocular Therapeutix and Mosaic focuses on the discovery and development of novel complement inhibitors with extended duration of activity. Under the terms of the agreement, Ocular Therapeutix has agreed to fund the research performed under the collaboration and retains all program inventions and associated intellectual property.
Approvals for newer therapies
Approvals for newer therapies can drive growth of North America & Europe age-related macular degeneration therapeutics market. On September 22, 2020, Gyroscope Therapeutics Limited, a U.K.-based clinical-stage retinal gene therapy company, announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation to GT005 for the treatment of geographic atrophy (GA) secondary to dry age-related macular degeneration (AMD). GT005 is an investigational gene therapy that is delivered under the retina and is intended to slow the progression of GA that can lead to blindness. Fast Track designation was granted to GT005 for the treatment of people with GA who have specific mutations in their Complement Factor I (CFI) gene and low levels of the CFI protein in their blood.
North America & Europe Age-Related Macular Degeneration Therapeutics Market: Restraints
High cost of treatment
The high cost of treatment is expected to hamper the North America & Europe age-related macular degeneration therapeutics market growth. For instance, according to an article published by the Biomolecules on November 3, 2022, while Anti-VEGF agents have provided a favorable prognosis for nAMD, they are associated with a substantial financial burden for patients and the healthcare system, due to their high cost as well as the need for frequent repeat treatments and visits. Ranibizumab portal delivery system (RPDS) used in age-related macular degeneration with fixed 6-months refills over a one-year duration cost US$ 21,016. The monthly intravitreal injections of ranibizumab cost US$ 1943, aflibercept cost US$ 5702, and bevacizumab cost US$ 16,732. Such high cost of medication can restrain market growth.
To counterbalance this restraint, a reimbursement policies should be introduce to reduce overall cost of treatment.
Side effects of wet AMD treatment
Side effects of wet AMD treatment is expected to hamper the North America & Europe age-related macular degeneration therapeutics market growth. For instance, according to an article published in the Journal of Ophthalmic and Vision Research on October 25, 2021, there are various wet AMD medications that have a variety of side effects. Effects including retinal vasculitis, intraocular inflammation, concurrent vascular occlusion, nausea, vomiting, and permanent vision loss in individuals are anticipated. Few reported side effects like lenticular opacities, punctate keratitis, corneal abrasion, posterior capsule opacification, cataract, increased intraocular pressure, blepharitis, conjunctivitis, and iritis. This inhibits market expansion.
To counterbalance this restrain, more research should be carried out to reduce side effects associated with treatment.
North America & Europe Age-Related Macular Degeneration Therapeutics Market- Key Players
Major players operating in the North America & Europe age-related macular degeneration therapeutics market include Novartis AG, Bayer AG, Bausch Health Companies Inc., Regeneron Pharmaceuticals, Inc., F. Hoffmann-La Roche AG, Pfizer, Inc., Valeant Pharmaceuticals International, Inc., AbbVie Inc., Viatris Inc., and Amgen Inc.
*Definition: Age-related macular degeneration (AMD), also known as macular degeneration, is a condition that results in loss of central vision due to the thinning of the macula of the retina. Age-related macular degeneration typically occurs in the geriatric population and can cause permanent vision loss in people aged 60 years and above. Age-related macular degeneration is of two types; dry age-related macular degeneration (Dry AMD) and wet age-related macular degeneration (wet AMD). Dry age-related macular degeneration is a common form of macular degeneration, where the macula gets thinner with age and leads to loss of central vision. There is no treatment available for dry age-related macular degeneration. Wet age-related macular degeneration is less common, where the growth of abnormal blood vessels is observed under the retina. Wet age-related macular degeneration can be managed through anti-vascular endothelial growth factor (anti-VEGF) injections.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients