Noninvasive prenatal testing (NIPT) is an evolving screening procedure for pregnant women. It examines cell free Deoxyribonucleic Acid (DNA) of both fetus and the mother. It provides an analysis of genetic screening using only one tube blood. NIPT provides predictions about the absence or presence of genetic abnormality in the fetus. It involves a simple method of blood withdrawal but presents high quality effectiveness for the detection of trisomy by minimizing the number of performing further invasive testing procedures. NIPT is performed to detect certain genetic abnormalities such as missing or extra copies of X and Y chromosomes, trisomy 18, trisomy 13, and Down syndrome. With technological advancements in the near future, NIPT will be able to detect other genetic abnormalities.
North Africa non-invasive prenatal testing market is estimated to be valued at US$ 2,796 million in 2021 and is expected to exhibit a CAGR of 8.7% over the forecast period (2021-2028).
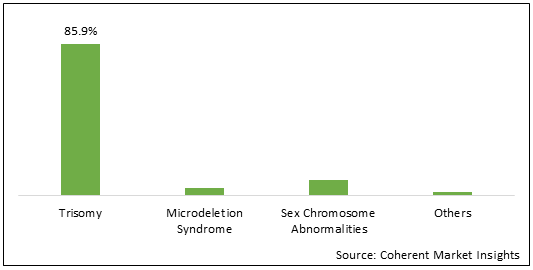
Figure 1. North Africa Non-invasive Prenatal Testing Market Share (%), By Application, 2021
To learn more about this report, Download Free Sample
The increasing adoption of inorganic strategies such as strategic collaborations is expected to drive the growth of North Africa non-invasive prenatal testing market over the forecast period.
For instance, in June 2017, Illumina, Inc., an American company, collaborated with Next Generation Genomic Co., Ltd., the Association of Southeast Asian Nations (ASEAN) leaders in laboratory services and reproductive science. This alliance will allow large patient access to Illumina under next-generation sequencing for NIPT.
North Africa Non-invasive Prenatal Testing Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2020 | Market Size in 2021: | US$ 2,796 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2021 to 2028 |
| Forecast Period 2021 to 2028 CAGR: | 8.7% | 2028 Value Projection: | US$ 4,759 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Illumina, Inc., Eurofins Scientific, PerkinElmer, Inc., F. Hoffmann-La Roche AG, Natera, Inc., Yourgene Health Plc., Laboratory Corporation of America Holdings (LabCorp), BGI Group, and Quest Diagnostics Incorporated |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
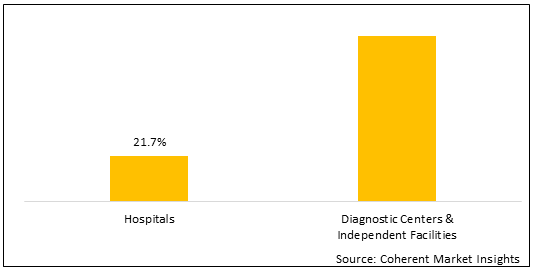
Figure 2. North Africa Non-invasive Prenatal Testing Market Share (%), By End User, 2021
To learn more about this report, Download Free Sample
Increasing product launches are expected to drive the market growth over the forecast period.
For instance, in May 2017, Natera Inc., a clinical genetic testing company, launched ‘Vistara’, a non-invasive prenatal test (NIPT). It is designed to screen single-gene disorders.
North Africa Non-invasive Prenatal Testing Market – Impact of Coronavirus (COVID-19) Pandemic
The COVID-19 outbreak was first reported on December 31, 2019 in Wuhan, China. According to the Coronavirus (COVID-19) Weekly Epidemiological Update by the World Health Organization (WHO), over 265,713,467 cases and 5,260,888 deaths due to the coronavirus were reported till December 07, 2021 across the globe.
The COVID-19 pandemic and subsequent lockdown in various countries have negatively impacted the financial status of businesses across all sectors. The COVID-19 pandemic has impacted the entire supply chain of the healthcare industry mainly due to strict lockdown in several regions. Likewise, it is expected to have a significant impact on North Africa non-invasive prenatal testing (NIPT) market, in terms of both demand and supply.
Society for Maternal-Fetal Medicine (SMFM) suggests that, the number of ultrasound appointments in the first trimester can be reduced by performing one ultrasound examination for dating and nuchal translucency (NT) to reduce the chances of COVID-19 exposure. NIPT can be performed at home utilizing remote services such as mobile blood draw networks. This will help maintain expectant mother’s safety during the pandemic with minimum social contact.
North Africa Non-invasive Prenatal Testing Market Restraint
The presence of alternative methods for prenatal diagnosis is expected to be a major factor hindering the North Africa non-invasive prenatal testing market growth over the forecast period.
For instance, amniocentesis and chorion biopsy are two alternative methods that are used in prenatal diagnosis. Amniocentesis is a medical test used for prenatal diagnosis and sex determination. Chorion biopsy, also known as Chorionic villus sampling (CVS), is another diagnostic test performed during pregnancy to determine if an unborn child is at risk of congenital defects.
NIPT based on cell-free DNA analysis from maternal blood is a screening test with certain chances of false positive and negative results. Furthermore, NIPT does not screen birth defects such as open neural tube defects or other conditions such as autism. NIPT tests also do not screen for polyploidy (e.g. triploidy) or single gene disorders. Such limitations are expected to hamper the growth of North Africa non-invasive prenatal testing market.
Key Players
Major players operating in North Africa non-invasive prenatal testing market include Illumina, Inc., Eurofins Scientific, PerkinElmer, Inc., F. Hoffmann-La Roche AG, Natera, Inc., Yourgene Health Plc., Laboratory Corporation of America Holdings (LabCorp), BGI Group, and Quest Diagnostics Incorporated.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients