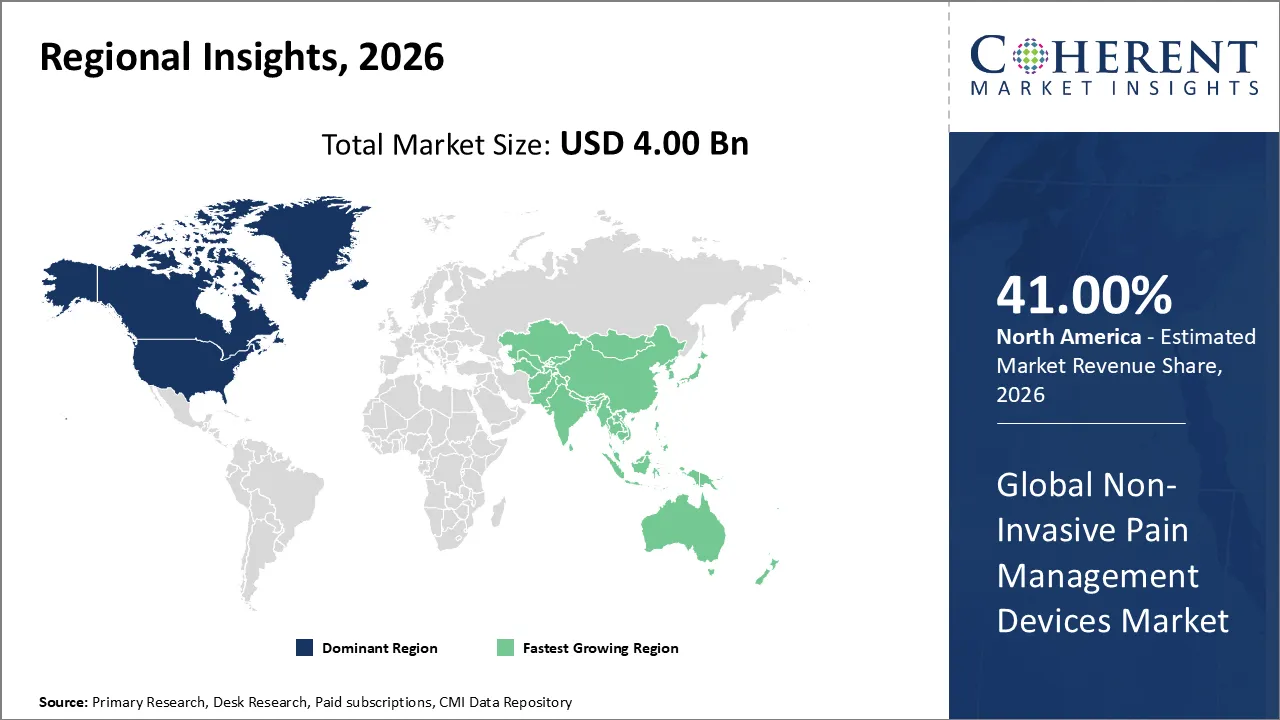
Non-invasive pain management devices market is estimated to be valued at USD 4.00 Bn in 2026 and is expected to reach USD 8.7 Bn in 2033, exhibiting a compound annual growth rate (CAGR) of 11.8% from 2026 to 2033.
The non-invasive pain management devices market is evolving rapidly, driven by growing demand for safe, drug-free solutions to chronic pain. Devices such as TENS units, wearable neurostimulation systems, ultrasound therapy, and cold/heat therapy tools are increasingly adopted in clinical and homecare settings. Rising awareness of opioid risks, technological innovation, and patient preference for portable, user-friendly options are fueling expansion. Integration with digital health platforms and personalized treatment approaches further strengthen the role of these devices in modern pain care.
|
Current Event |
Description and its Impact |
|
Regulatory Framework Evolution |
|
|
Technological Advancement and Innovation |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
AI is revolutionizing the non-invasive pain management devices market by enabling personalized, adaptive, and data-driven therapies. Through advanced algorithms, devices can analyze patient-specific pain patterns, predict episodes, and adjust stimulation in real time. This improves treatment outcomes, reduces opioid dependence, and expands home-based care. AI integration also supports remote monitoring, telehealth, and digital health ecosystems, making pain management more accessible and cost-effective. As adoption grows, AI-powered neuromodulation and neurostimulation are expected to drive significant market expansion and reshape chronic pain care globally.
For instance, in September 2025, the Australian-first launch of Nevro® HFX AdaptivAI™ highlights AI’s role in non-invasive pain management devices. Using real-time data from thousands of patients, it personalizes spinal cord stimulation to treat chronic pain. This breakthrough demonstrates how AI-driven neurostimulation is reshaping pain care, offering safer, adaptive alternatives to opioids.
In terms of product type, the electric stimulation devices segment is expected to hold 57% share in 2026, due to their affordability, portability, and proven effectiveness in managing chronic pain. Widely prescribed for arthritis, back pain, and neuropathic conditions, they offer drug-free relief and are increasingly integrated with digital health platforms for personalized therapy.
For instance, in January 2026, The FDA's approval of the first brain stimulation device for depression that can be used at home indicates the significance of non-invasive electrical stimulation technologies. It is mostly for mental health, but it uses the same neurostimulation principles as pain management. This suggests that drug-free, portable devices can be used in many different types of modern therapeutic care.
In terms of application, the musculoskeletal pain segment is expected to lead the market with 42% share in 2026, driven by the high prevalence of arthritis, lower back pain, and sports injuries. Patients prefer non-invasive solutions that provide consistent relief without medication risks. The broad patient base and clinical acceptance make musculoskeletal pain the largest contributor to device adoption worldwide.
For instance, in December 2025, Curapod's release of India's first personalized wearable for drug-free pain relief is an important advance forward in managing pain without surgery. The portable device targets musculoskeletal conditions like arthritis, back pain, and sports injuries. It offers personalized therapy that meets patients' needs for safe, consistent relief, which is driving the rapid growth of the Asia-Pacific market.
In terms of end user, the hospital segment is projected to account 47% share in 2026, supported by advanced infrastructure, physician oversight, and reimbursement frameworks. They handle complex pain cases requiring specialized equipment and clinical validation. Hospitals also serve as primary hubs for introducing new technologies, ensuring widespread adoption of non-invasive pain management devices across patient populations.
For instance, in September 2025, the launch of the CryoXTTM device by AtriCure for pain after surgery is an example of the way that non-invasive hospital care is getting better. It lowers surgical pain without using opioids, which is in line with clinical validation and doctor supervision.
To learn more about this report, Download Free Sample
North America is expected to dominate the non-invasive pain management devices market with 41% share in 2026, due to high chronic pain prevalence, strong healthcare infrastructure, and widespread adoption of advanced technologies. Robust reimbursement frameworks, physician awareness, and patient preference for drug-free therapies further drive demand, positioning the region as the leading hub for innovation and usage.
For instance, in November 2025, The Neurolyser XR HIFU system is now available at Sault Area Hospital in Ontario, Canada, for managing pain without surgery. It uses focused ultrasound to target nerves without surgery or drugs. This new technology makes North America even more powerful in 2026, as hospitals lead the way in using advanced, drug-free methods to treat complex pain.
Asia Pacific is expected to exhibit the fastest growth, due to its vast patient pool, rising prevalence of arthritis and diabetes-related neuropathy, and growing awareness of drug-free therapies. Expanding healthcare infrastructure, government initiatives, and affordability of portable devices further accelerate adoption, making the region the fastest-growing market globally.
For instance, in May 2025, Matri has released India's thinnest dual-mode period pain relief device, which can only be found on MyMatri.com. It is made for women's health and uses advanced wearable technology to give drug-free, non-invasive relief. This innovation highlights Asia-Pacific’s rapid growth in non-invasive pain management, expanding applications beyond musculoskeletal pain into personalized solutions for menstrual discomfort.
The U.S. dominates the Non-Invasive Pain Management Devices Market in 2026 due to high chronic pain prevalence, advanced healthcare infrastructure, and strong reimbursement policies. Widespread adoption of innovative technologies, physician awareness, and patient preference for drug-free therapies drive demand, positioning the country as the global leader in this sector.
For instance, in October 2025, Boston Scientific's acquisition of Nalu Medical, Inc., which is based in California, adds to its neuromodulation portfolio in North America. Nalu's main focus is on peripheral nerve stimulation for chronic pain, which is a drug-free and minimally invasive way to relieve pain.
China dominates the Non-Invasive Pain Management Devices Market in 2026 due to its vast patient population, rising prevalence of chronic pain, and strong demand for drug-free therapies. Rapid healthcare infrastructure expansion, government support, and affordability of wearable neuromodulation devices drive adoption, making China the fastest-growing hub in Asia-Pacific.
For instance, in September 2025, The China NMPA approved Pier 88 Health and Theranica permission to proceed for the Nerivio REN wearable, a drug-free neuromodulation device that helps treat acute migraines. This important step forward in non-invasive pain management in the Asia-Pacific region shows how China is becoming a leader in using new, personalized treatments that cut down on the need for medication and surgery to relieve pain.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 4.00 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 11.8% | 2033 Value Projection: | USD 8.7 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Orbasone Pain Relief System (Orthometrix, Inc), Medio UniSono eco (Iskra Medical), Medio MULTI eco (Iskra Medical), ActivBody, Apostherapy (Sportsmed), VitalStim Plus (DJO Global) etc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The growing incidence of osteoporosis, arthritis, back pain, neuropathic pain, and migraine is significantly fueling the adoption of advanced therapies. Chronic pain affects millions worldwide, creating a sustained need for safe, long-term solutions that avoid invasive procedures. This trend directly boosts the non-invasive pain management devices market share, as patients and healthcare providers increasingly rely on wearable neuromodulation, TENS, and other drug-free technologies to manage pain effectively across diverse age groups and geographies. For instance, around 20% of Americans, which is nearly 50 million people are reported experiencing chronic pain. By 2023, this already high prevalence rose to more than 24%, adding another 10 million individuals. As a result, over 60 million people nationwide were living with chronic pain, underscoring its growing and widespread impact on public health.
Concerns about opioid addiction, dependency, and adverse side effects are accelerating the move toward non-invasive, non-pharmacological alternatives. Governments and healthcare providers are actively promoting non-opioid strategies, encouraging adoption of electrical stimulation and wearable neuromodulation devices. This shift is driving strong non-invasive pain management devices market demand, as patients seek safer, portable, and personalized solutions that reduce reliance on medication while improving quality of life in both clinical and home-care settings.
Neuromodulation and neurostimulation are central pillars of innovation in the non-invasive pain management devices market forecast, offering effective alternatives to pharmaceuticals and invasive procedures. Neuromodulation works by altering nerve activity through targeted electrical or magnetic stimulation, reducing pain perception in conditions like migraines, neuropathic pain, and musculoskeletal disorders. Neurostimulation, on the other hand, applies controlled electrical impulses to nerves or the spinal cord to block pain signals before they reach the brain, with popular methods including transcutaneous electrical nerve stimulation (TENS). Together, these approaches are driving rapid adoption of portable, user-friendly, and home-based devices, positioning them as high-growth opportunities in the global market.
The market for non-invasive pain management devices is growing steadily, driven by rising prevalence of chronic pain and growing preference for drug-free ways to treat it. The global demand shows a shift toward technologies that provide targeted, easy-to-use solutions while reducing the need for oral medications and invasive procedures. Hospitals, clinics, physiotherapy centers, and home care settings are all important end-use segments. This shows that these devices are becoming more widely used and accepted.
Electrotherapy devices, especially TENS units, are still very important in the market because they work and are easy to use. These devices are commonly used to relieve musculoskeletal, neuropathic, and general pain. New features like wearable formats, built-in sensors, and mobile app connectivity are making it easier to customize treatment. Other treatments, like neuromuscular electrical stimulation (NMES) and low-level laser therapy, are becoming more popular in rehabilitation, sports medicine, and post-operative recovery. This shows that they can be used for more than just pain management.
North America has the highest rate of regional adoption, due to its healthcare infrastructure, reimbursement systems, and high level of consumer awareness. The Asia-Pacific markets are growing quickly as more people are able to get healthcare, people are spending larger amounts of cash, and more people are interested in alternative ways to relieve pain. In general, the market is moving toward devices that are technologically advanced, have multiple functions, and put the patient first. These devices should be safe, effective, and easy to use for managing pain in both clinical and home care settings.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients