Non-Invasive Cancer Diagnostics Market Size and Forecast – 2025 – 2032
The Global Non-Invasive Cancer Diagnostics Market size is estimated to be valued at USD 8.45 billion in 2025 and is expected to reach USD 18.70 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 12.5% from 2025 to 2032.
Global Non-Invasive Cancer Diagnostics Market Overview
Non-invasive cancer diagnostic products encompass liquid biopsy kits, imaging modalities, and molecular testing devices that detect cancer biomarkers without surgical intervention. Liquid biopsy tests analyze circulating tumor DNA (ctDNA), exosomes, and other biomarkers from blood or urine samples to identify cancer presence and progression. Imaging products, such as advanced MRI and PET systems, allow real-time visualization of tumors. Innovations in next-generation sequencing (NGS) and AI-assisted diagnostic tools are enhancing accuracy, early detection, and personalized treatment monitoring.
Key Takeaways
The liquid biopsy segment dominates the technology landscape, commanding a 43% market share due to its clinical efficacy and patient compliance benefits.
Lung cancer remains the largest cancer type segment, reflecting epidemiological trends and robust funding for early detection solutions targeting this indication.
Blood-based sample diagnostics lead in adoption due to ease of access and broad applicability across cancer types.
North America accounts for the largest industry share, benefiting from a strong market ecosystem, advanced healthcare infrastructure, and favorable government initiatives.
Asia Pacific emerges as the fastest-growing region, registering a CAGR exceeding 14%, driven by increasing healthcare expenditure, rising cancer incidence, and expanding diagnostic infrastructure.
Non-Invasive Cancer Diagnostics Market Segmentation Analysis
To learn more about this report, Download Free Sample
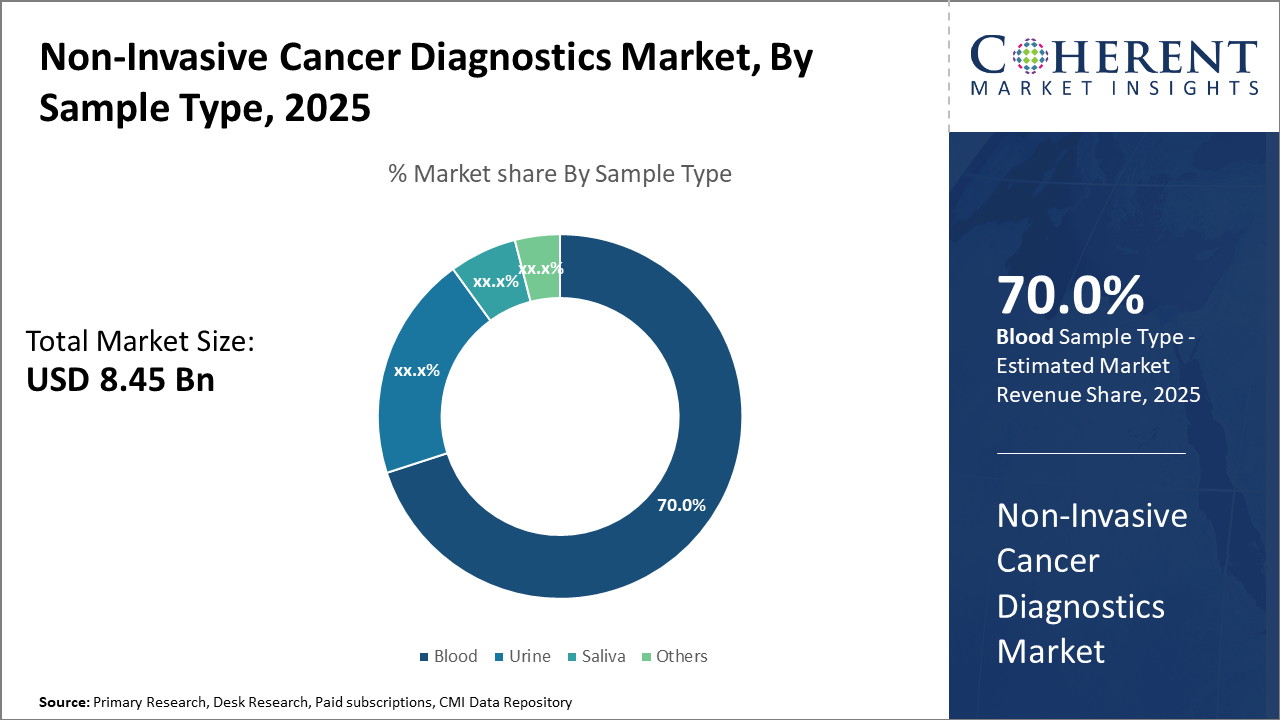
Non-Invasive Cancer Diagnostics Market Insights, By Sample Type
Blood sample-based diagnostics remain the benchmark for most non-invasive tests, especially liquid biopsies analyzing circulating tumor cells and ctDNA, accounting for over 70% of the sample type market share. Urine-based diagnostics are fastest fastest-growing, benefiting from novel biomarker discoveries relevant for urological and bladder cancers, offering a non-invasive alternative that can be self-collected. Saliva samples are increasingly leveraged in head and neck cancer monitoring due to ease of collection and emerging molecular assays.
Non-Invasive Cancer Diagnostics Market Insights, By Treatment Type
Liquid Biopsy dominates the market share, due to its minimally invasive nature and high specificity in detecting ctDNA, making it the preferred choice for continuous cancer monitoring and early diagnosis. Its rising clinical acceptance is propelled by studies confirming its sensitivity exceeding 85% for various solid tumors. Imaging Diagnostics continues to play a critical role, especially in structural tumor visualization and staging, while innovations in PET and MRI scans are enhancing resolution and reducing scan times. Molecular Diagnostics, encompassing tests like PCR and NGS, supports targeted therapy decisions with genomic profiling.
Non-Invasive Cancer Diagnostics Market Insights, By Cancer Type
Lung Cancer diagnostics lead largely due to the high incidence and mortality rate necessitating early detection solutions, with non-invasive methods now responsible for detecting nearly 60% of cases prior to clinical symptoms. Breast Cancer diagnostics are the fastest-growing subsegment, driven by increased screening programs and advanced genomic assays enhancing personalized treatment. Colorectal Cancer non-invasive tests, including fecal DNA testing, are gaining traction owing to their ease of use and accuracy. Prostate Cancer diagnostics benefit from PSA testing improvements and new biomarker discovery.
Non-Invasive Cancer Diagnostics Market Trends
The non-invasive cancer diagnostics market is heavily influenced by technological innovation and regulatory support, favoring early cancer detection.
In 2025, the adoption of liquid biopsy has surged primarily due to its utility in real-time tumor profiling and monitoring, reflected by a 43% market share within the technology segment. AI-powered diagnostic tools have also moved from experimental stages to clinical application, improving diagnostic accuracy and reducing false positives, thereby enhancing physician confidence.
A significant market shift is observed in the Asia Pacific, where government initiatives in countries like China and India aim to increase cancer screening rates, supported by local manufacturing of diagnostic tools to reduce costs.
For example, China’s recent health policy encourages integration of molecular diagnostics in routine oncology, contributing to the Asia Pacific’s CAGR surpassing 14%.
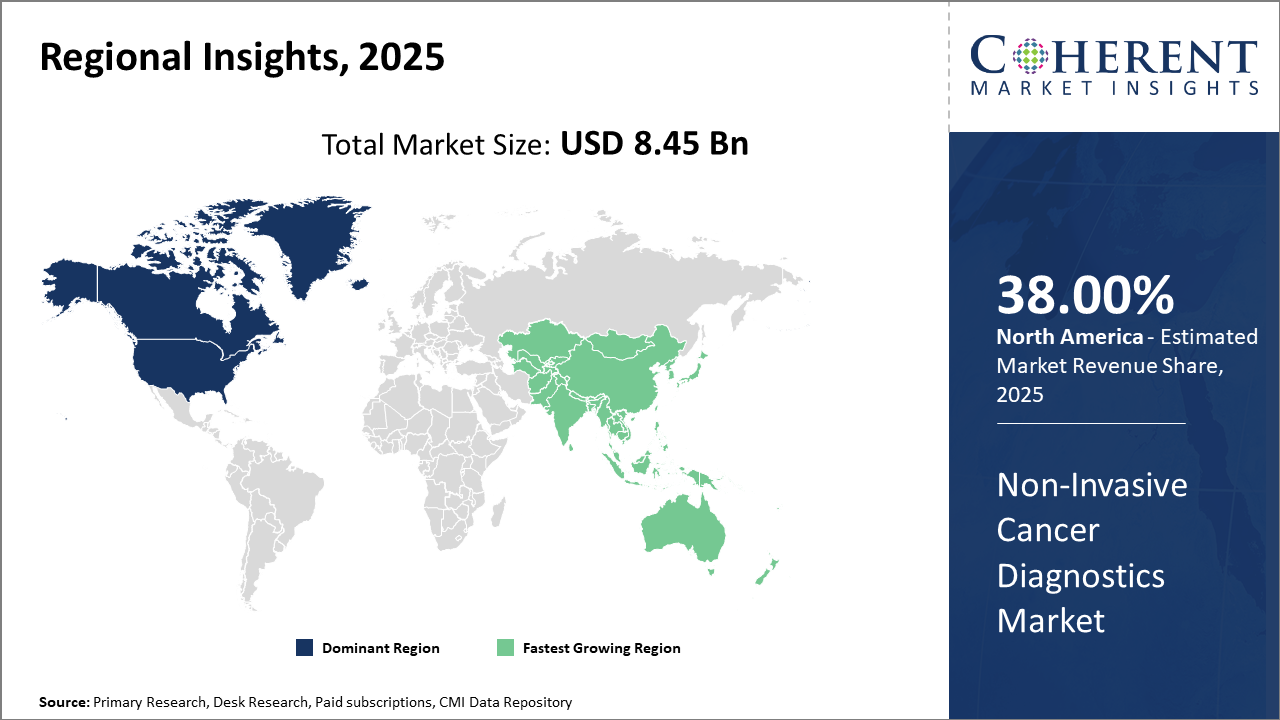
Non-Invasive Cancer Diagnostics Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Non-Invasive Cancer Diagnostics Market Analysis and Trends
In North America, the dominance in the Non-Invasive Cancer Diagnostics market is attributed to an advanced healthcare ecosystem, widespread clinical adoption, and favorable insurance reimbursement policies. The region contributes over 38% of the global market share. Companies like Guardant Health and Exact Sciences, headquartered in the U.S., play critical roles in innovation and commercialization, maintaining strong pipelines for liquid biopsy and molecular diagnostic tests.
Asia Pacific Non-Invasive Cancer Diagnostics Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth with a CAGR above 14%, driven by increasing cancer incidence, improving healthcare infrastructure, and government schemes enhancing accessibility to diagnostics. Emerging market players are capitalizing on domestic demand with cost-effective solutions, while multinational companies focus on strategic alliances to penetrate this expanding market.
Non-Invasive Cancer Diagnostics Market Outlook for Key Countries
USA Non-Invasive Cancer Diagnostics Market Analysis and Trends
The USA’s market is pivotal, propelled by aggressive federal funding increases for cancer diagnostics totaling over USD 1.6 billion in 2024 alone. Key players such as Guardant Health and Illumina deploy cutting-edge liquid biopsy and NGS technologies extensively across oncology clinics. Consequently, the U.S. serves as a robust innovation hub, setting clinical standards that influence global non-invasive cancer diagnostics trends.
China Non-Invasive Cancer Diagnostics Market Analysis and Trends
China's market benefits from national cancer prevention strategies, with substantial investment in local molecular diagnostic manufacturing. Leading domestic companies have formed partnerships with international firms to enhance technology transfer and regulatory approvals. This synergy has escalated China’s market penetration considerably, contributing to Asia Pacific's status as the fastest growing region in this market.
Analyst Opinion
The rising adoption of liquid biopsy technology is a key quantitative driver accelerating market growth. Recent clinical studies published in 2024 indicate that liquid biopsy techniques accounted for approximately 43% of diagnostic testing in oncology, up from 32% in 2023, establishing them as a dominant segment due to their minimal invasiveness and ability to detect circulating tumor DNA (ctDNA) with high sensitivity.
Demand-side dynamics, such as escalating investments in early cancer detection programs, have directly influenced market size. For instance, government bodies in major markets like the U.S. increased funding for non-invasive diagnostic technologies by 24% in 2024 compared to 2023, highlighting supportive policies that contribute to market revenue expansion.
Supply-side indicators involving pricing strategies reveal an upward trend in premium diagnostic tests. In 2025, pricing for next-generation sequencing (NGS) based diagnostic panels saw a 15% increase, yet adoption remained strong due to better reimbursement schemes and growing healthcare provider confidence.
Micro-indicators, including advancements in biomarker discovery, have ushered in new test panels targeting early-stage detection, particularly for lung and colorectal cancers. Clinical trials associated with novel biomarkers expanded by 38% in 2024, fostering diversification of non-invasive diagnostic portfolios and enhancing market growth.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 8.45 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 12.5% | 2032 Value Projection: | USD 18.7 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Guardant Health, Exact Sciences, Illumina, Inc., Roche Diagnostics, Foundation Medicine, Inc., Qiagen N.V., Biocept, Inc., Grail, Inc., Thermo Fisher Scientific, Sysmex Corporation, Myriad Genetics, Inc., Bio-Rad Laboratories, Inc. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Non-Invasive Cancer Diagnostics Market Growth Factors
Increasing cancer incidence globally has significantly driven the demand for non-invasive diagnostic tools, particularly in regions with aging populations. According to recent epidemiological data in 2025, cases of lung and colorectal cancer increased by 9.3% compared to 2023, heightening the need for early diagnostic solutions. Rising patient preference for outpatient procedures is another crucial driver, propelling the adoption of liquid biopsy and imaging diagnostics due to their minimal discomfort and rapid results. The expansion of reimbursement policies favoring advanced diagnostics, notably in North America and Europe, has lowered financial barriers, facilitating adoption. Additionally, continuous technological innovation in biomarker identification and digital pathology has enhanced test accuracy, making non-invasive diagnostics a preferred choice among market players and healthcare providers alike.
Non-Invasive Cancer Diagnostics Market Development
In October 2025, MiRXES received regulatory approval in China to launch its groundbreaking blood-based diagnostic test for gastric cancer, marking a major milestone in early cancer detection. The test, developed using MiRXES’s proprietary microRNA (miRNA) biomarker technology, enables the non-invasive detection of early-stage gastric cancer through a simple blood draw.
In June 2025, Dxcover Limited announced the launch of its U.S. operations to commercialize its AI-powered multiomic cancer detection platform, marking a significant step in the company’s global expansion strategy. The move into the U.S. market follows successful clinical validation studies demonstrating the test’s ability to distinguish between healthy and cancerous samples across various tumor types with high accuracy.
Key Players
Leading Companies of the Market
Guardant Health
Exact Sciences
Illumina, Inc.
Roche Diagnostics
Foundation Medicine, Inc.
Qiagen N.V.
Biocept, Inc.
Grail, Inc.
Thermo Fisher Scientific
Sysmex Corporation
Myriad Genetics, Inc.
Bio-Rad Laboratories, Inc.
Several leading companies have implemented aggressive growth strategies, such as strategic acquisitions and partnerships to broaden their cancer diagnostic portfolios. For example, Illumina’s acquisition of Grail in 2024 led to enhanced offerings in early cancer detection, increasing the company’s market share by 8%. Roche Diagnostics expanded collaborations with biotech firms to fast-track liquid biopsy approvals, achieving a 22% year-over-year revenue increase in this segment.
Non-Invasive Cancer Diagnostics Market Future Outlook
In the future, the Non-Invasive Cancer Industry is expected to experience rapid expansion as technologies converge to deliver highly sensitive, multi-cancer screening tools. Advances in liquid biopsy, next-generation sequencing, and AI-assisted imaging will enable earlier intervention and real-time monitoring of disease progression. Companies will focus on decentralizing diagnostic testing to reach broader patient populations through at-home and point-of-care solutions. Strategic collaborations among biotech firms, hospitals, and data analytics providers will shape the competitive landscape. Regulatory clarity, along with reimbursement expansion, will further support widespread integration into clinical practice.
Non-Invasive Cancer Diagnostics Market Historical Analysis
The non-invasive cancer diagnostics market emerged from the growing need to detect malignancies earlier and more accurately without surgical procedures. Initially limited to imaging modalities like MRI, CT, and ultrasound, technological progress introduced molecular diagnostics, biomarker assays, and liquid biopsy platforms capable of identifying cancer through blood, saliva, or urine samples. Integration of bioinformatics, genomics, and data analytics transformed oncology diagnostics into a precision science, enhancing sensitivity and clinical relevance. The shift toward patient-centric care, combined with healthcare digitization and government initiatives for early cancer detection, bolstered global adoption.
Sources
Primary Research interviews:
Oncologists
Diagnostic Pathologists
Medical Device Engineers
Biotech Executives
Databases:
NCI Cancer Research Data
WHO Cancer Statistics
Magazines:
Diagnostics World
Medical Device Network
Cancer Therapy Advisor
Nature Biotechnology
Journals:
Cancer Research
Journal of Clinical Oncology
Oncotarget
Analytical Chemistry
Newspapers:
The Washington Post (Health)
The Economic Times (Healthcare)
The Guardian (Science)
The New York Times (Health)
Associations:
American Cancer Society
World Health Organization
International Agency for Research on Cancer (IARC)
American Association for Cancer Research
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients