The Global Nitinol Medical Devices Market is estimated to be valued at USD 1.99 Bn in 2025 and is expected to reach USD 4.03 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 10.6% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
Nitinol has emerged as an effective material for developing minimally invasive medical devices and implants due to its shape memory effect and biocompatibility. Major factors fueling the adoption of nitinol medical devices include rising aging population worldwide, increasing preference for minimally invasive surgeries, and growing incidence of target diseases such as cardiovascular diseases. Moreover, ongoing technological advancements such as the development of advanced nitinol alloys with improved mechanical and corrosion resistant properties are further expanding the application areas of these devices. Key players in the market are focused on new product launches and geographic expansion strategies to capitalize on growth opportunities.
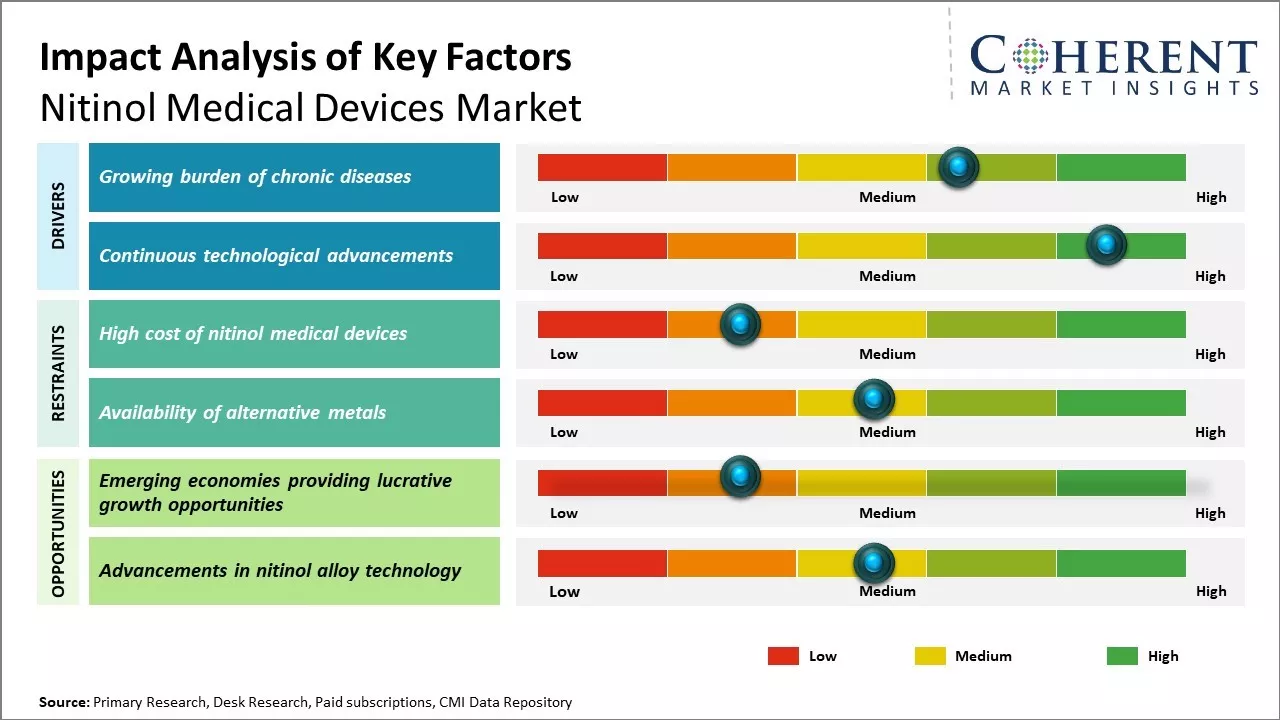
Growing burden of chronic diseases
The global nitinol medical devices market is poised for growth, driven by the increasing burden of chronic diseases like cardiovascular diseases (CVDs). The rise in prevalence of conditions such as coronary and peripheral vascular disease is boosting the adoption of self-expanding nitinol stents, which have transformed the treatment of peripheral vascular disease. With approximately 6.5 million people aged 40 and older in the U.S. affected by peripheral artery disease, market growth is expected to surge. In 2021, the World Health Organization (WHO) reported that approximately 17.9 million people succumbed to cardiovascular disease (CVD) annually, constituting 32% of global fatalities. Of these, 85% resulted from heart attacks and strokes. Moreover, procedures like angioplasty, which involve the insertion of balloons or stents into blood vessels, have driven the utilization of nitinol medical devices. Nitinol is predominantly used in the manufacturing of stents, grafts, balloons, guiding guidewires, and catheters employed during these procedures.
Get actionable strategies to beat competition: Download Free Sample
Continuous technological advancements
Continuous innovation in the nitinol medical devices market is driven by the development of miniature and sophisticated devices. Nitinol, a nickel-titanium alloy, offers unique properties like shape memory effect and pseudo-elasticity, enabling the creation of flexible yet durable medical components. Manufacturers leverage these properties for minimally invasive procedures, facilitated by advancements such as 3D printing. This technology allows for the design of intricate geometries, leading to localized drug delivery stents and enhanced healing abilities through nano structuring. Additionally, bioabsorbable components are being developed to eliminate the need for multiple implant removals post-treatment. Medical, imaging advancements enable precise pre-surgical planning, with 3D organ models aiding device sizing and finite element analysis simulating stresses on devices and tissues. For instance, in January 2022, Cook Medical, a medical device company, was granted Breakthrough Device designation by the U.S. Food and Drug Administration (FDA) for a novel drug-eluting stent intended for below-the-knee (BTK) use. This innovative stent is developed to address the needs of patients suffering from chronic limb-threatening ischemia (CLTI).
Key Takeaways of Analyst:
The nitinol medical devices market has strong growth potential over the coming years. North America currently dominates due to favorable reimbursement policies and rising healthcare spending. The market is expected to transition towards the Asia Pacific region as quality healthcare infrastructure expands across developing nations.
Key drivers of market growth include the increasing prevalence of target diseases and conditions and rising number of invasive surgeries performed globally. Nitinol's biocompatible and self-expanding properties make it well-suited for applications such as vascular stents and bone fracture repair. Its superior flexibility and shape memory abilities allow for minimally invasive procedures.
However, high production costs pose a challenge as nitinol is an expensive raw material to procure and work with. Substitutes exist that are less costly but may not provide the same clinical benefits. Stringent regulatory approvals also lengthen the time taken to commercialize new nitinol-based products.
Nevertheless, ongoing R&D into newer applications will further cement nitinol's importance in domains like interventional cardiology, orthopedics and neurology over the next decade. Focus on specialty stents, guidewires and sutures can unlock exciting opportunities.
Market Challenges: Availability of alternative metals
The global nitinol medical devices market faces challenges due to the availability of alternative metals such as cobalt-chromium alloys and stainless steel, which are also used for medical devices. Additionally, copper-based alloys are gaining popularity due to their lower cost. Other metals like gold, silver, platinum, iridium, tungsten, and tantalum are also utilized in medical devices, further impacting nitinol market growth. Moreover, the high manufacturing costs associated with nitinol devices compared to materials like stainless steel hinder market expansion, despite the numerous advantages of nitinol.
Market Opportunities: The increasing adoption of nitinol-based medical products worldwide
The global nitinol medical devices market is poised for growth due to increased adoption of nitinol-based products worldwide. For instance, Confluent Medical's expansion plans include setting up a pilot manufacturing facility for nitinol products in India and Asia. Additionally, the formation of regulatory guidelines, such as the FDA's draft guidance in April 2019, presents significant growth opportunities for market players.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
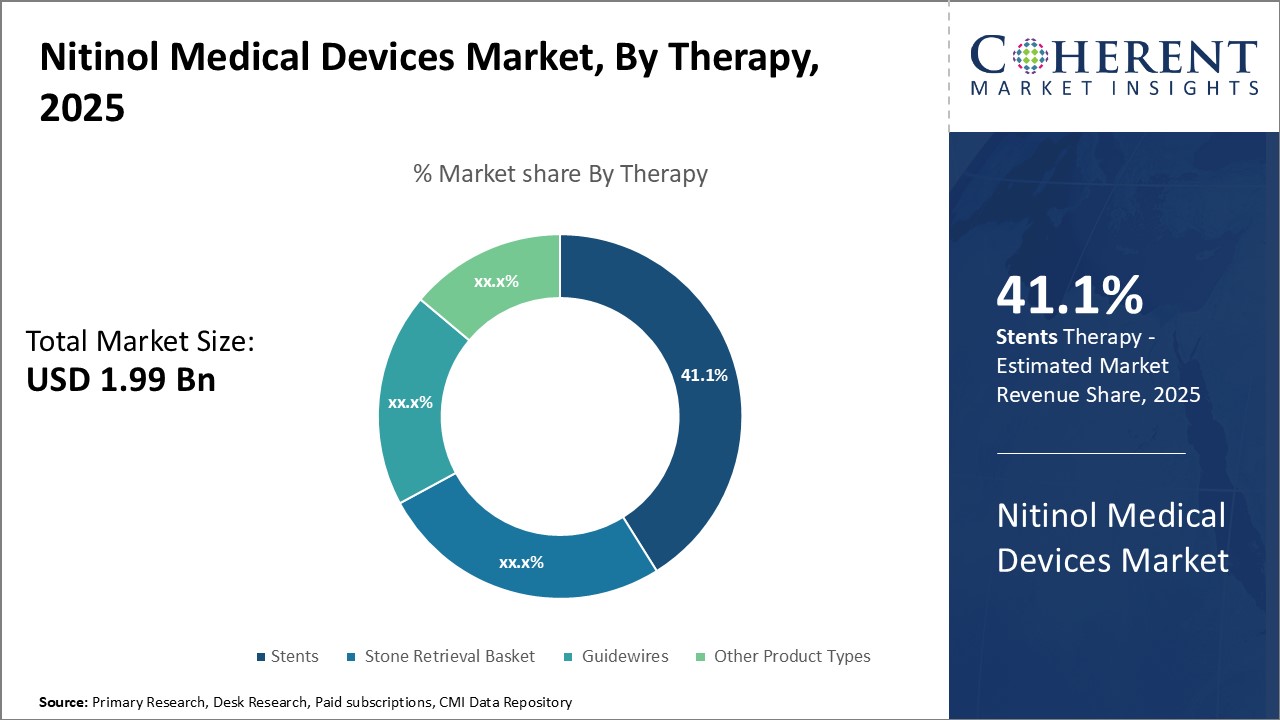
In terms of Therapy Type, Stents holds the highest share of the market owing to their increasing usage rates and technological advancements
Stents have emerged as one of the most widely used medical devices for treating various cardiovascular diseases with highest share of 41.1% in 2025. It’s made from the shape-memory alloy nitinol, stents are highly flexible and kink-resistant, making them easier for physicians to maneuver through veins and arteries. Their self-expanding property also means they require minimal manipulation and force to deploy at the target site.
The use of stents has grown significantly over the past decade due to the rise in cardiac conditions like atherosclerosis and coronary artery disease. As the global population ages, the incidence of such diseases is increasing at an alarming rate. Stents provide minimally invasive treatment options for patients who are not surgical candidates or who require an alternative to open-heart surgeries. Compared to other treatments, stents have shown better recovery times and success rates.
In terms of Application, Cardiovascular holds the highest share of the market driven by a growing elderly population and rising incidence of heart disease
The cardiovascular segment dominates the nitinol medical devices market and is estimated to hold a market share of 59.62% in 2025, due to the widespread use of nitinol-based products in treating heart and vessel conditions. Coronary artery disease (CAD), a leading cause of mortality worldwide, is a significant driver in this segment. With increasing access to healthcare and aging populations, demand for interventional cardiology procedures involving nitinol devices continues to rise. Other cardiac disorders, such as arrhythmias, heart failure, valvular heart disease, and congenital heart defects, also contribute to this segment's growth. Nitinol devices like pacemakers, implantable cardioverter-defibrillators (ICDs), and heart valves help manage irregular heart rhythms and repair damaged valves or congenital abnormalities. Additionally, rising obesity levels contribute to opportunities in treating vascular abnormalities with nitinol stents, embolic coils, and vena cava filters.
Need a Different Region or Segment? Download Free Sample
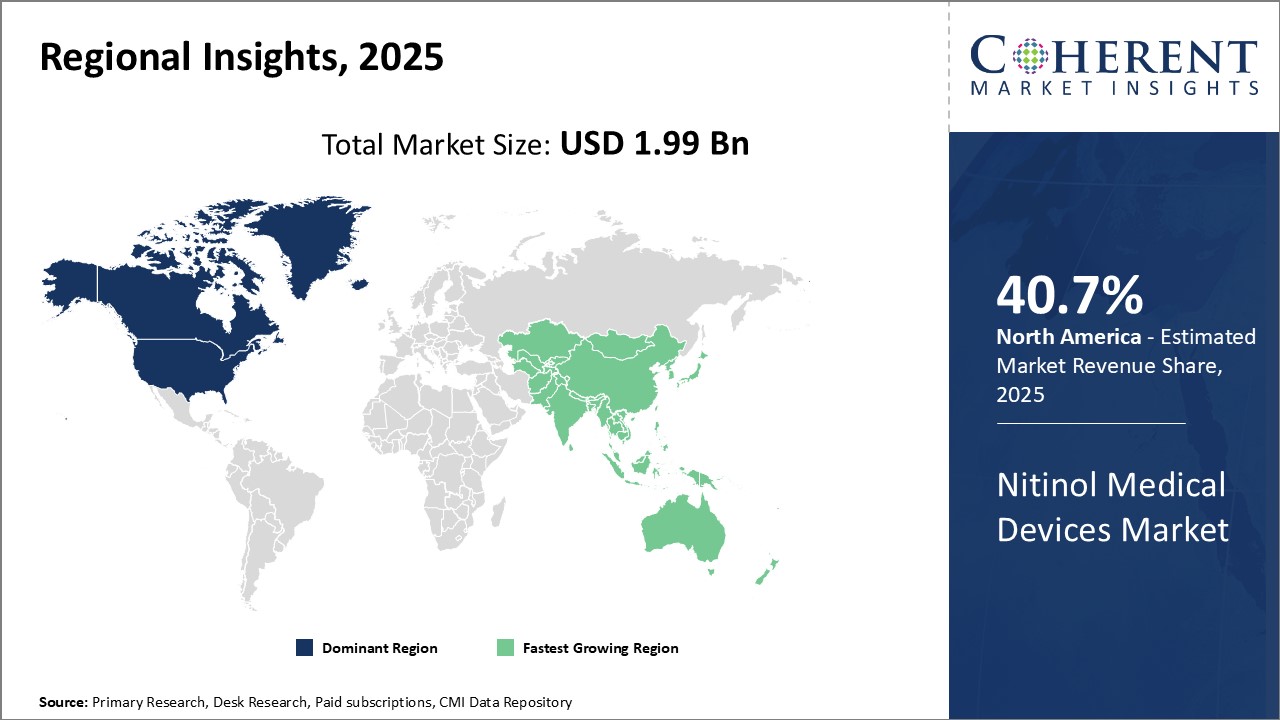
The U.S. has dominated the global nitinol medical devices market for several years now with share of 40.7% in 2025. Factors driving the large U.S. market share include strong domestic demand from the country's large population and prominent biomedical industry presence. The U.S. is home to many top medical device companies that are global leaders in developing and commercializing Nitinol-based products. These companies invest heavily in R&D efforts to bring new and improved Nitinol solutions to the market. They also benefit from attractive reimbursement policies set by the U.S. government.
In addition, the U.S. has a favorable regulatory environment for new medical technologies. The approval pathway through the FDA is seen as more predictable compared to other major regions. This enables U.S. companies to get their Nitinol innovations approved and launched faster, maintaining the region's competitive edge. Nitinol raw material suppliers also have a significant presence in North America, making key inputs more accessible and ensuring security of domestic supply chains. For these reasons, the U.S. market is a dominant source of global demand for Nitinol devices.
Nitinol Medical Devices Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1.99 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 10.6% | 2032 Value Projection: | USD 4.03 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
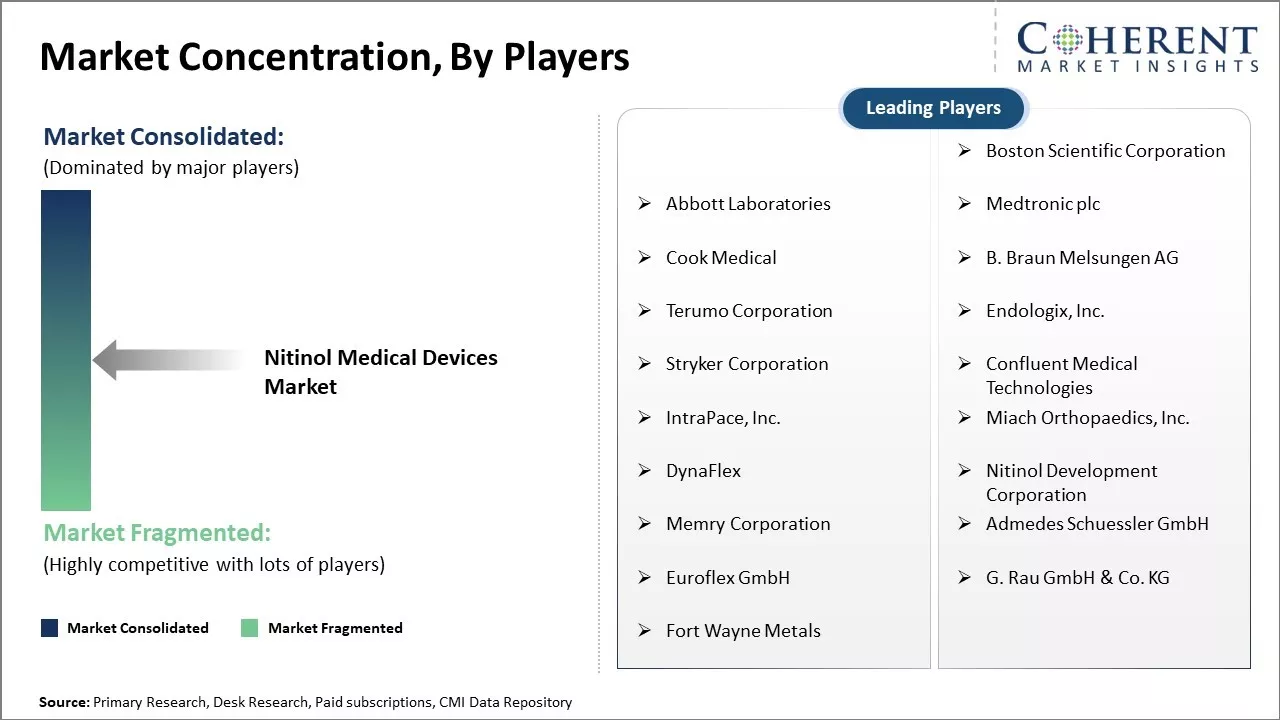
Boston Scientific Corporation, Abbott Laboratories, Medtronic plc, Cook Medical, B. Braun Melsungen AG, Terumo Corporation, Endologix, Inc., Stryker Corporation, Confluent Medical Technologies, IntraPace, Inc., Miach Orthopaedics, Inc., DynaFlex, Nitinol Development Corporation, Memry Corporation, Admedes Schuessler GmbH, Euroflex GmbH, G. Rau GmbH & Co. KG, and Fort Wayne Metals |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: The Nitinol Medical Devices market involves the development and use of advanced simulation technology and devices for training medical professionals. It allows doctors, nurses and other healthcare personnel to practice procedures, treat complications and communicate with simulated patients in a safe, controlled environment before working with real people. Nitinol Medical Devices aims to improve clinical skills through experiencing lifelike clinical scenarios and receiving feedback, with the goal of enhancing patient safety, outcomes and the overall quality of healthcare.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients